The 2023 International evidence‐based guideline for the assessment and management of polycystic ovary syndrome project was led and primarily funded by Australia through the National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL).1,2,3,4,5,6,7 Responding to strong calls from patient and consumer groups, the guideline addresses a condition that affects one in eight Australian women, 140 million women globally, and is complex, underdiagnosed, undertreated, underfunded, under‐researched, misnamed and misunderstood.1,2,3,4,5,8,9,10,11 As a neglected women's health condition, polycystic ovary syndrome (PCOS) is relegated to brief education snapshots (usually under infertility), or no dedicated content in undergraduate health professional curricula. Individuals affected report limited knowledge and dismissive attitudes from health professionals (Box 1), despite the high prevalence, broad clinical features and marked impact on quality of life.1,2,3,4,5,6 PCOS is also a misnomer as it is neither an isolated disease of the ovaries or involving true ovarian cysts.5,11 Rather, it is a genetic and lifestyle‐related chronic endocrine condition with insulin resistance and hyperandrogenism and a myriad of reproductive, psychological, cardiometabolic and dermatological features.6,12,13 To address these challenges and in response to both patient and health professional priorities, we aimed to engage internationally and develop and translate comprehensive evidence‐based guidelines for diagnosis, assessment and treatment, to improve the lives of individuals with PCOS worldwide. Here, we outline the process and key recommendations, applying an Australian lens.
Methods
Guideline development
The guideline development process was overseen by international advisory and management committees, guideline development groups (GDGs) and paediatric, consumer and translation committees. Participants were nominees of CRE WHiRL, international co‐funding partners (the American Society for Reproductive Medicine, the Endocrine Society, the European Society of Endocrinology and the European Society of Human Reproduction and Embryology) and 35 collaborating professional and consumer organisations, including ten from Australia.14 Guideline scope and clinical question prioritisation were derived from consumer input based on the public participation spectrum framework,15 formative research,9,16 a global survey of more than 750 women,14 and involved experts.17
Evidence scoping and allocation
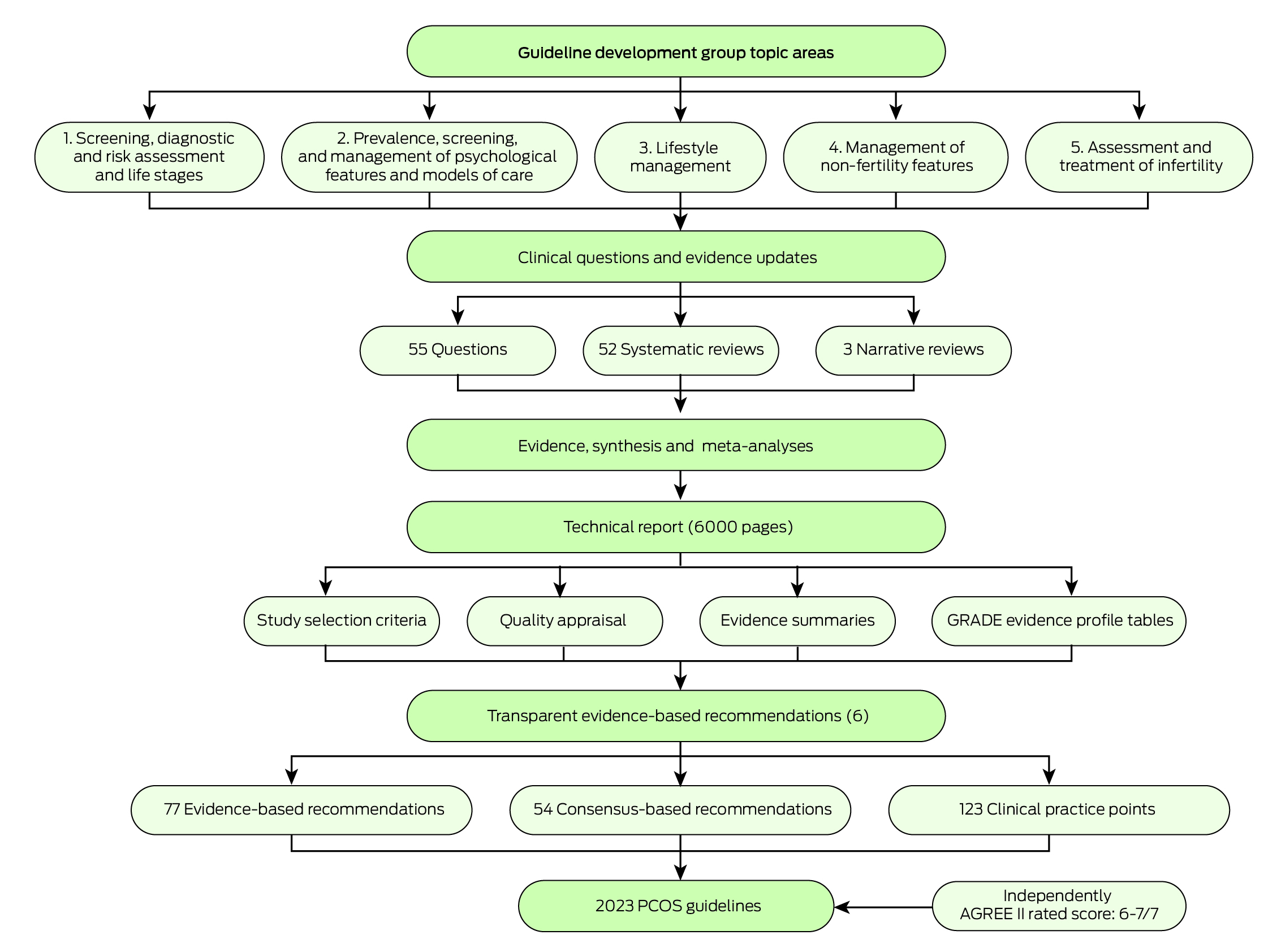
The evidence team, led by AM and CTT and supported by HJT, established, trained, supervised and mentored 26 early‐career researchers in a network recruited from engaged organisations and GDG affiliations to undertake systematic reviews.17 Guideline methods aligned with best practice NHMRC requirements and the Appraisal of Guidelines for Research and Evaluation‐II (AGREE‐II) tool18 as documented in the full guideline and technical report.1,2,3,4 Comprehensive evidence synthesis involved 52 systematic reviews and generated new evidence from extensive meta‐analyses. Study inclusion was determined from a priori population, intervention/exposure, comparison, outcome (PICO/PECO) frameworks.6 Databases (including CINAHL and MEDLINE, MEDLINE In‐Process and other non‐indexed citations, PsycINFO, EMBASE and All EBM Reviews via OVID) were searched for published, English language articles. Titles, abstracts, keywords and full texts were screened and the evidence team, GDG clinical leads and evidence experts were engaged throughout (Box 2).6
Study‐level method quality was assessed with a risk of bias rating of low, moderate, high or insufficient information. Evidence certainty was determined at the outcome‐level using Grading of Recommendations, Assessment, Development and Evaluation (GRADE) evidence to decision framework,19 across risk of bias, inconsistency, indirectness, imprecision and other bias (eg, publication bias) as documented in the technical report.6 Critical outcomes were determined by GDG clinical leads and consumers and influenced overall certainty of evidence.
To ensure authenticity and accuracy of evidence, the Research Integrity in Guidelines and evIDence synthesis (RIGID) framework20 was developed, with study level integrity scores using the TRACT tool,21 and an integrity committee for consensus on study allocations. Authors of studies with moderate and high risk of integrity concerns were contacted. All scores and reasons were tabulated in the technical report.6 Data were extracted into evidence summary templates, with meta‐analyses done using Review Manager or STATA. Statistical heterogeneity was assessed using I2 and explored via sensitivity analysis and subgroup analyses, where possible.
Clinical leads drafted recommendations, refined at face‐to‐face meetings using the GRADE framework, across certainty, quality, feasibility, acceptability, cost and implementation. With clinical and consumer input, recommendations and strengths were determined. The evidence quality was improved from the 2018 guidelines but was generally low–moderate quality. Overall, the guideline development process resulted in 52 systematic reviews and analyses, 77 evidence‐based and 54 consensus recommendations, with 123 practice points. The draft guideline was externally reviewed via public and targeted consultation of engaged organisations, including consumer groups. Adaptations occurred in response to national and international public consultation and peer review. After meeting the standard for clinical practice guidelines, the guideline was approved by NHMRC in July 2023 (Box 2).
Recommendations
Diagnosis and history
The updated recommendations emphasise the importance of diagnosing PCOS according to international, evidence‐based guideline criteria,22 building on consensus‐based Rotterdam criteria.23 The presence of at least two of the following points is required in adults: (i) clinical or biochemical hyperandrogenism, (ii) ovulatory dysfunction or irregular cycles, and (iii) polycystic ovarian appearance or morphology (PCOM) identified through ultrasound or anti‐Müllerian hormone (AMH) levels. For women with irregular cycles and hyperandrogenism (approximately 70% of cases), ultrasound (practice point; PP) or AMH level testing (evidence‐based recommendation; EBR) is not required for diagnosis. For women who only have either irregular menstrual cycles or hyperandrogenism, pelvic ultrasound or the newly added AMH level testing (but not both), is needed for adult PCOS diagnosis (PP) (Supporting Information, figure 1).1,2,3,4,24,25 Biochemical testing for hyperandrogenism is only needed without clinical hyperandrogenism (PP). For adolescents, both hyperandrogenism and ovulatory dysfunction are necessary for diagnosis, with both pelvic ultrasound (PP) and AMH level testing (EBR) not recommended due to lack of specificity in the pubertal transition.1,2,3,4
Although the absence of core diagnostic features makes initial PCOS diagnosis post‐menopause difficult, retrospective diagnosis is possible based on clinical features and, together with a historical diagnosis, can be considered enduring (consensus recommendation; CR).1,2,3,4 Importantly, it is now recommended that individuals with PCOS be recognised as having increased cardiovascular disease risk factors, cardiovascular disease and potentially cardiovascular mortality (EBR).6 Potential increased cardiometabolic risk in first‐degree family members including diabetes, metabolic syndrome and hypertension were also recognised (EBR for male family members, PP for female family members). High risks of early onset impaired fasting glucose, impaired glucose tolerance and type 2 diabetes in PCOS were reiterated (EBR), while high risks of obstructive sleep apnoea (EBR) and endometrial hyperplasia and cancer (EBR) were noted. Evidence‐based screening recommendations are outlined in Supporting Information, figure 2.1,2,3,4 Metabolic features exist independent of, but are exacerbated by, excess weight (EBR).1,2,3,4
New recommendations highlight significantly higher risks in pregnancy, independent of age and body mass index (BMI), including early miscarriage, excess gestational weight gain, gestational diabetes, hypertension in pregnancy, preeclampsia, small for gestational age babies, preterm delivery and increased incidence of caesarean delivery (EBR). Health professionals should ensure that PCOS status is identified preconception or early in antenatal care, and women with PCOS should be identified, screened and monitored before and during pregnancy for prevention and treatment (EBR).1,2,3,4
Adolescent perspective
The guideline used the World Health Organization definition of adolescence, which defines it to be the period between ten and 19 years, which coincides with physiological changes in puberty including menstrual irregularities, hyperandrogenism and PCOM. These physiological changes overlap with adult PCOS diagnostic criteria, thus affecting diagnostic accuracy.9,26,27 Although delayed diagnosis is commonly reported in adults, many adults also note a missed diagnosis in adolescence.9 The 2018 and 2023 international guidelines updated the Rotterdam consensus diagnostic criteria to evidence‐based guideline criteria. These include recommendations to avoid using non‐specific features common in adolescence (defining PCOM by ultrasound [PP] or by AMH level [EBR]), to promote accurate diagnosis during this life stage and more clearly defining each diagnostic component1,2,3,4 (Supporting Information, figure 1). Recognising the risk of underdiagnosis, the guideline also recommends identification of individuals with only one diagnostic criterion (hyperandrogenism or irregular cycles) as being “at risk of PCOS” and requiring follow‐up (PP).1,2,3,4 Adolescents with one criterion have greater weight gain, more metabolic and hyperandrogenic features than healthy adolescents, but less than individuals meeting both criteria, warranting reassessment (PP).
Timely diagnosis allows for identification and prevention in individuals who are at high risk of long term complications, before potential long term use of contraception, which can mask PCOS symptoms.26,28 In adolescents “at risk” or diagnosed with PCOS, the guideline recommends combined oral contraceptive pill (COCP) alone or metformin alone (EBR). Evidence in adolescents with PCOS is relatively scarce with limited research to date.28 Notably, the prevalence of depressive symptoms and depression in adolescents with PCOS is high (EBR), yet management is often suboptimal with dissatisfaction reported in education, information and emotional support.27
Psychological health and wellbeing
All health professionals should be aware that PCOS carries increased risks of negative psychosocial outcomes and mental health conditions, including depression, anxiety, eating disorders, disordered eating, body image distress, low self‐esteem and problems with feminine identity (EBR).1,2,3,4,29,30 These affect the quality of life (EBR) and likely the capacity to engage in PCOS‐related lifestyle interventions and self‐management (PP). Mental health guidelines rarely recognise PCOS as being associated with psychological conditions. Despite this, most people with PCOS report that these outcomes are under‐recognised, with limited emotional support and counselling highlighting a need for increased awareness and reduced stigma regarding mental health symptoms (CR).1,2,3,4 Treatment of other clinical features (eg, acne or hirsutism) can improve quality of life (PP) and, alongside psychological support (CR), needs to be prioritised.6
The guideline recommends that depression and anxiety are routinely screened for in PCOS (EBR), given the high prevalence.1,2,3,4 Screening for depression and anxiety is common practice in Australia, particularly in the Better Access Initiative. Commonly used tools include the Depression, Anxiety Stress Scale31 and the Kessler Psychological Distress Scale.32 The Better Access Initiative facilitates up to ten individual and ten group Medicare‐rebated sessions for mental health conditions. The guideline recommends that eating disorders and disordered eating are considered, regardless of weight, especially in the context of weight management and lifestyle interventions (EBR).1,2,3,4 Although routine screening is not recommended, an assessment should be completed when eating disorders or disordered eating are suspected,1,2,3,4 with the Eating Disorders Examination Questionnaire33 commonly used in Australia, particularly in eating disorder treatment and management plans (PP). Individuals with mild to moderate eating disorders may be eligible for ten Better Access sessions (up to ten individual and up to ten group sessions per year), individuals with severe eating disorders may be eligible for up to 40 evidence‐based psychological sessions, and up to 20 dietetic sessions annually. The guideline does not recommend routine screening, but does recommend that patients with body image distress, low self‐esteem, problems with feminine identity, or psychosexual dysfunction be offered evidence‐based treatments (eg, cognitive behavioural therapy) where appropriate (CR). Individualised approaches focusing on personal priorities are recommended.1,2,3,4
The guideline recommends psychological therapy be considered first‐line (CR), and that women with depression, anxiety or eating disorders should be offered evidence‐based psychological therapy,34,35,36,37,38 as per general population guidelines and patient preferences (CR). Mental health interventions can be effectively delivered via multiple modalities including face‐to‐face, telephone, videoconference and online, including guided self‐help interventions. Numerous high quality, Australian mental health support resources are available, including e‐Mental Health in Practice, Head to Health, Black Dog Institute, Beyond Blue, Butterfly Foundation, Centre for Clinical Interventions, and This Way Up.
Models of care, quality information provision and shared decision making
Consumer input highlighted the critical need for the provision of high quality information (EBR) and for established interdisciplinary and patient‐centred PCOS models of care with acknowledgement of unique experiences, concerns and goals (CR),6,39 and adaptation for cultural and health care system factors. Health care professionals need to shift from traditional decision makers to empowering shared decision making and self‐management (EBR). The models of care must build on evidence‐based practice and necessitates multidisciplinary collaboration (CR).40,41 In Australia, there are few dedicated models of care, except for the Victorian PCOS service, which aligns with guidelines and improves patient experience.41 The Victorian Government has recognised the need and is expanding services, whereas most other states and territories remain underserved. Enhanced health professional education, systematically embedded at all levels of health professional training, is recommended to address PCOS knowledge gaps (CR). Recommendations also target research and policy sectors to advance knowledge and improve care and health outcomes. Provision of comprehensive and accurate information is paramount for shared, informed decision making,42 and to enhance knowledge and self‐management.42 Health care professionals have a vital role in engaging in open and respectful communication and providing guidance and support in individualised person‐centred care.
Lifestyle management
Higher weight gain and higher weight are common features of PCOS with a two‐fold higher risk of having overweight, obesity or central obesity.43 Adverse lifestyle factors (higher energy intake, nutrition with high glycaemic index, increased sitting time and lower physical activity) have greater impact in PCOS, compared with controls.44 Lifestyle management should be recommended to all individuals with PCOS (EBR) and encompass both prevention of weight gain, and support for individuals with overweight or obesity (CR). Healthy lifestyle behaviours encompassing healthy eating and physical activity should be recommended to optimise general and metabolic health, and improve quality of life (CR),1,2,3,4 noting benefits even without weight loss (PP). Within lifestyle interventions, behavioural strategies may increase engagement (PP).1,2,3,4 Importantly, for dietary and exercise components, there is no one superior approach (EBR)45 with practice guided by general population guidelines46,47 and individual preferences and goals (CR), and referral to allied health professionals where required (PP).1,2,3,4 Other healthy eating patterns recommended in national and international evidence‐based guidelines for healthy eating, diabetes or cardiovascular disease prevention or management include the Mediterranean diet and the Dietary Approaches to stop Hypertension (DASH) diet.48 Misinformation on fad diets should also be refuted and evidence‐based information provided.
Co‐developed, realistic and tailored lifestyle goals are key (PP), acknowledging that weight stigma and negative biopsychosocial impacts are common in PCOS (PP). Weight‐neutral care could be considered where warranted or preferred with further research needed (PP).49 Health care professionals and policy makers need to recognise that the primary drivers of excess weight sit at a policy, environmental and social level, with 70% of Australians above healthy weight. This surpasses isolated individual behavioural lifestyle change and mandates broader population level strategies.
Management of non‐fertility features
Medications for PCOS are generally off‐label, or prescribed for an indication or patient group not specified in approved product information by the regulatory body, despite evidence of efficacy. This is unavoidable and common, usually due to lack of industry funded submissions to regulatory bodies, which are less likely with off‐patent medications used in PCOS. COCPs are recommended in adults with PCOS or adolescents with or at risk of PCOS, for irregular cycles or hirsutism (EBR).1,2,3,4,50 Low‐dose preparations are recommended (≤ 30 μg of oestradiol)50,51,52 for treating hirsutism in adults, with limited additional benefit with cyproterone acetate containing agents, which are second‐line treatments, due to side effects (EBR).51 COCP use should align with general population guidelines and balance efficacy, metabolic risk, side effects, cost and availability (EBR).53 Mechanical laser and light therapies should be considered for reducing hirsutism and for related depression, anxiety and quality of life in PCOS (EBR), ideally delivered by experienced practitioners (CR). In combination with effective contraception, anti‐androgens could be considered to treat hirsutism in women with PCOS, if there is a suboptimal response after a minimum of six months of COCP and/or cosmetic therapy (EBR).1,2,3,4,54
Metformin should be considered in adults with PCOS and a BMI ≥ 25 kg/m2 for anthropometric and metabolic outcomes including insulin resistance, and glucose and lipid profiles (EBR).55 Metformin can be considered for irregular cycles where the COCP is contraindicated (PP) or in adolescents (EBR).52,55 Metformin should be considered over inositol for hirsutism and central adiposity, noting increased side effects with metformin (EBR). Inositol (in any form) may be considered in PCOS based on individual preferences and values, given limited harm, potential for reduced biochemical hyperandrogenism and metabolic measures, yet with limited evidence for clinical ovulation, hirsutism or weight benefits (EBR).
There is limited research on anti‐obesity medications in PCOS. However, there is evidence in the general population allowing use with active lifestyle for weight management in adults with PCOS, as per population guidelines (CR), ensuring effective contraception given limited pregnancy safety data (PP).1,2,3,4 In Australia, regulatory status is evolving but currently Pharmaceutical Benefits Scheme reimbursement of newer agents is limited to diabetes management and access can be challenging. Bariatric surgery can be considered for women with PCOS for weight loss, hypertension, diabetes prevention and treatment, irregular cycles and pregnancy rates, informed by population guidelines (CR).56 Fertility can return soon after surgery, and pregnancy is not recommended until weight stability, requiring interim contraception (CR). Access and out‐of‐pocket costs are ongoing considerations. Finally, research into these interventions in PCOS was prioritised within the guidelines and the research priorities map.1,2,3,4
Screening, diagnostic assessment and management of infertility
Women with PCOS should be reassured that pregnancy will often be successfully achieved either naturally or with oral ovulation induction (PP), especially with early PCOS diagnosis and a focus on prevention. Fertility treatment in PCOS should be guided by the fertility treatment algorithm (PP) (Supporting Information, figure 3).1,2,3,4 Prenatal vitamin supplementation should be started (PP) (including folate, which needs to be given at a higher dose in individuals with a BMI > 30 kg/m2) and routine preconception care instituted (CR), to optimise comorbid conditions such as diabetes and high blood pressure (PP). Women should be counselled on the adverse impact of excess weight on fertility, miscarriage and live birth rates (EBR), while avoiding weight stigma and considering cultural, social and environmental determinants of health (PP). Blood pressure, smoking, alcohol, diet, nutritional status, exercise, sleep, mental, emotional and sexual health should be considered and optimised (CR).1,2,3,4
The risks, benefits, costs, timing and techniques of tubal patency testing before starting ovulation induction should be considered on an individual basis in the presence of anovulation with normal semen analysis (CR). Use of ovulation induction agents is off‐label in Australia but allowed, and health care professionals need to inform women and discuss evidence, concerns and side effects (PP). Letrozole should be the first‐line pharmacological treatment for ovulation induction in infertile anovulatory women with PCOS with no other infertility factors (EBR). Other less effective ovulation induction agents, such as metformin, clomiphene citrate and combinations thereof, may be considered following explanation of benefits, risks, efficacy and costs (EBR).1,2,3,4
Either gonadotrophins (low dose) or laparoscopic ovarian surgery could be second‐line treatment options for anovulatory and infertile PCOS, with clomiphene citrate resistance and no other infertility factors (EBR), following counselling on higher live birth rate and higher multiple pregnancy rates with gonadotrophins, and considering expertise, monitoring, risks and costs. In the absence of an absolute indication for in vitro fertilisation or intracytoplasmic sperm injection, in vitro fertilisation could be offered in women with PCOS and anovulatory infertility, if first‐ or second‐line ovulation induction therapies fail (CR). Alternatively, in vitro maturation can be considered with adequate expertise, acknowledging less effectiveness for cumulative live birth rate but better potential safety for ovarian hyperstimulation syndrome (EBR).1,2,3,4 These recommendations are applicable to the Australian health care system, including pre‐pregnancy care usually provided by general practitioners and secondary fertility care involving specialist referral.
Translation
Recommendations are supported by evidence‐based, freely accessible co‐designed resources for health professionals (algorithms, webinars, toolkits and training programs) and patients (ASKPCOS app reaching 196 countries and more than 60 000 women is available at https://mchri.org.au/guidelines‐resources/community/askpcos‐app/; fact sheets [Supporting Information, figure 4], booklets and webinars, in multiple languages and available at https://mchri.org.au/guidelines‐resources/community/pcos‐resources/). Co‐development of a best practice framework to guide models of care and of sophisticated digital tools are underway for interactive, shared decision making. Evaluation includes benchmarking models of care and ongoing assessment of the Australian Longitudinal Study of Women's Health data on diagnosis, screening and treatment aligned to guideline recommendations nationally. Translation is prioritised and funded and will include broad engagement with relevant stakeholders. This will include Indigenous Australians, with higher PCOS prevalence and more limited access to care in regional, rural and remote areas.
Conclusion
The 2023 guideline targets recommendations towards training organisations, policy makers, research funders, health professionals and individuals with PCOS. Overall, the guideline responds to limited education, inadequate research and models of care and takes a strong patient‐centric approach to support women with PCOS and health professionals in Australia and globally. The guideline process has generated a research priorities map to guide future research and translation. The guideline also emphasises the need for a best practice framework for effective models of care and for greater medical and health professional undergraduate and postgraduate education, with work currently underway in this area. For individuals with PCOS, improved knowledge transfer and optimised shared decision making is important. We encourage all stakeholders to recognise this common, neglected condition and to implement evidence‐based recommendations for improving diagnosis and care experiences and optimising health outcomes for the one in eight Australian women affected by PCOS.
Box 1 – Lived experience of PCOS
Lorna Berry, who has polycystic ovary syndrome (PCOS), was one of the lived experience experts on the guideline. Here she shares her journey, highlighting the challenges in obtaining a diagnosis and reliable information, which greatly impacted her wellbeing, mental health and fertility options. She firmly believes that no woman should go undiagnosed and unsupported.
I fought for my PCOS diagnosis, being repeatedly dismissed by health professionals. PCOS affected all aspects of my life, family, fertility, wellbeing and lifestyle. Living with PCOS is challenging enough, but the struggle to find reliable information feels like an uphill battle. It's disheartening when every corner you turn, there's someone trying to sell a miracle cure. I need trustworthy information that deals with my concerns and answers my questions and empathetic, knowledgeable health professionals who take this seriously to avoid the frustration and sense of hopelessness that can go with having PCOS. Empowering women so they can advocate and partner with and educate doctors and other health professionals is of utmost importance. We need to make sure that the next generation doesn't go undiagnosed and unsupported.
Provenance: Commissioned; externally peer reviewed.
- Helena J Teede1
- Aya Mousa1
- Chau T Tay1
- Michael F Costello2,3,4
- Leah Brennan5
- Robert J Norman6
- Alexia S Pena6
- Jacqueline A Boyle7
- Anju Joham1
- Lorna Berry8
- Lisa Moran1,6
- 1 Monash Centre for Health Research and Implementation, Monash University, Melbourne, VIC
- 2 University of New South Wales, Sydney, NSW
- 3 Royal Hospital for Women, Sydney, NSW
- 4 Monash IVF, Sydney, NSW
- 5 La Trobe University, Melbourne, VIC
- 6 Robinson Research Institute, University of Adelaide, Adelaide, SA
- 7 Monash University, Melbourne, VIC
- 8 Polycystic Ovary Syndrome Association of Australia, Sydney, NSW
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
This effort was primarily funded by the Australian Government via the National Health Medical Research Council (NHMRC) (APP1171592), supported by a partnership with the American Society for Reproductive Medicine, the Endocrine Society, the European Society for Human Reproduction and Embryology, and the European Society for Endocrinology. The Commonwealth Government of Australia also supported guideline translation through the Medical Research Future Fund (MRFCRI000266). Helena Teede (2009326) and Aya Mousa (1161871) are funded by NHMRC fellowships. Lisa Moran is funded by a Heart Foundation (101169) and a Veski Fellowship. Guideline development group members were volunteers. Travel expenses were covered by the sponsoring organisations. Disclosures of interest were strictly managed according to NHMRC policy and are available with the full guideline, technical evidence report, peer review and responses (www.monash.edu/medicine/mchri/pcos).
The evidence synthesis network was led by Chau Tay and Aya Mousa, across search strategies, training, Covidence processes, quality appraisal and GRADE, meta‐analysis, evidence integrity processes and preparing the technical report. The members of this network led evidence synthesis across the clinical questions, had input into the technical report and are listed in the technical report. Ben Mol and Madeline Flanagan (along with Aya Mousa, Michael Costello, Robert Norman, Chau Tay and Helena Teede) were members of the guideline integrity team.
We gratefully acknowledge contribution of our partners and collaborating organisations: the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL) (APP1171592), Centre for Research Excellence in Polycystic Ovary Syndrome (CRE PCOS) (APP1078444) and the members of these centres who coordinated this international guideline effort. Other collaborating societies and consumer groups providing in‐kind support are listed in the guideline. Other relevant organisations are welcome to apply to partner in guideline translation.
Of the named authors: Helena Teede, Aya Mousa, Chau Tay, Leah Brennan, Alexia Pena, Jacqueline Boyle, Lorna Berry and Lisa Moran have no conflicts of interest to declare. Michael Costello declares travels support from Merck; and sits on an advisory board for Merck. Robert Norman has received speaker's fees from Merck. Anju Joham has received speaker's fees from Novo Nordisk and Boehringer Ingelheim.
- 1. Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2023; 38: 1655‐1679.
- 2. Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2023; 120: 767‐793.
- 3. Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol 2023; 189: G43‐G64.
- 4. Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab 2023; 108: 2447‐2469.
- 5. Teede H, Gibson‐Helm M, Norman RJ, Boyle J. Polycystic ovary syndrome: perceptions and attitudes of women and primary health care physicians on features of PCOS and renaming the syndrome. J Clin Endocrinol Metab 2014; 99: E107‐E11.
- 6. Mousa A, Tay CT, Teede HJ. Technical report for the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Monash University, July 2023. https://bridges.monash.edu/articles/report/Technical_Report_for_the_2023_International_Evidence‐based_Guideline_for_the_Assessment_and_Management_of_Polycystic_Ovary_Syndrome/23625288?file=41455206 (viewed Aug 2024).
- 7. Teede HJ, Tay CT, Laven J, et al. International evidence‐based guideline for the assessment and management of polycystic ovary syndrome 2023. Monash University. Melbourne, Australia. 2023. https://www.monash.edu/medicine/mchri/pcos/guideline (viewed June 2024).
- 8. Gibson‐Helm M, Dokras A, Karro H, et al. Knowledge and practices regarding polycystic ovary syndrome among physicians in Europe, North America, and internationally: an online questionnaire‐based study. Semin Reprod Med 2018; 36: 19‐27.
- 9. Gibson‐Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102: 604‐612.
- 10. Brakta S, Lizneva D, Mykhalchenko K, et al. Perspectives on polycystic ovary syndrome: is polycystic ovary syndrome research underfunded? J Clin Endocrinol Metab 2017; 102: 4421‐4427.
- 11. Norman R, Mormon R, Teede H. “Tis but thy name that is my enemy”– the problem with the naming of PCOS. Fertil Steril 2023; 120; 249‐250.
- 12. Joham AE, Norman RJ, Stener‐Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol 2022; 10: 668‐680.
- 13. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev 2022; 43: 927‐965.
- 14. Teede HLJ, Moran L, Dokras A, et al. International evidence‐based guideline for the assessment and management of polycystic ovary syndrome 2018. Monash University. Melbourne, Australia. https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence‐Based‐Guidelines_20181009.pdf (viewed June 2024).
- 15. International Association for Public Participation. IAP2 Spectrum of Public Participation [website]. IAP2 International Federation; 2018. https://iap2.org.au/resources/spectrum/ (viewed Feb 2023).
- 16. Gibson‐Helm ME, Lucas IM, Boyle JA, Teede HJ. Women's experiences of polycystic ovary syndrome diagnosis. Fam Pract 2014; 31: 545‐549.
- 17. Tay CT, Garrad R, Mousa A, et al. Polycystic ovary syndrome (PCOS): international collaboration to translate evidence and guide future research. J Endocrinol 2023; 257: e220232.
- 18. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med 2010; 51: 421‐424.
- 19. Brozek JL, Akl EA, Alonso‐Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009; 64: 669‐677.
- 20. Mousa A, Flanagan M, Tay C, et al. Research Integrity in Guidelines and evIDence synthesis (RIGID): a framework for assessing research integrity in guideline development and evidence synthesis. eClinical Medicine 2024; 16 July: doi: 102717 [online ahead of print].
- 21. Mol BW, Lai S, Rahim A, et al. Checklist to assess Trustworthiness in RAndomised Controlled Trials (TRACT checklist): concept proposal and pilot. Res Integr Peer Rev 2023; 8: 6.
- 22. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; 33: 1602‐1618.
- 23. Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41‐47.
- 24. Anand S, Kumar A, Prasad A, Trivedi K. Updated meta‐analysis on the diagnostic accuracy of serum anti‐Mullerian hormone in poly cystic ovary syndrome involving 13 509 subjects. J Obstet Gynaecol Res 2022; 48: 2162‐2174.
- 25. Zhao Y, Zhao Y, Wang C, Liang Z, Liu X. Diagnostic value of anti‐Mullerian hormone as a biomarker for polycystic ovary syndrome: a meta‐analysis update. Endocr Pract 2019; 25: 1056‐1066.
- 26. Peña AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence‐based guideline. BMC Med 2020; 18: 72.
- 27. Peña AS, Teede H, Hewawasam E, et al. Diagnosis experiences of adolescents with polycystic ovary syndrome: cross‐sectional study. Clin Endocrinol (Oxf) 2022; 96: 62‐69.
- 28. Tay CT, Hart RJ, Hickey M, et al. Updated adolescent diagnostic criteria for polycystic ovary syndrome: impact on prevalence and longitudinal body mass index trajectories from birth to adulthood. BMC Med 2020; 18: 389.
- 29. Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta‐analysis. JAMA 2017; 317: 2207‐2225.
- 30. Davitadze M, Malhotra K, Khalil H, et al. Body image concerns in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Eur J Endocrinol 2023; 189: R1‐R9.
- 31. Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2nd ed. Sydney, Australia: Psychology Foundation, 1995.
- 32. Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non‐specific psychological distress. Psychol Med 2002; 32: 959‐976.
- 33. Fairburn CG, Beglin S. Eating disorder examination questionnaire (EDE‐Q 6.0). In: Fairburn CG. Cognitive behaviour therapy and eating disorders. New York, USA: Guildford Press; 2008; Appendix II.
- 34. Malhi GS, Bell E, Singh AB, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: major depression summary. Bipolar Disord 2020; 22: 788‐804.
- 35. Malhi GS, Bell E, Bassett D, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2021; 55: 7‐117.
- 36. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry 2018; 52: 1109‐1172.
- 37. Hay P, Chinn D, Forbes D, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust N Z J Psychiatry 2014; 48: 977‐1008.
- 38. Ralph AF, Brennan L, Byrne S, et al. Management of eating disorders for people with higher weight: clinical practice guideline. J Eat Disord 2022; 10: 121.
- 39. Barry MJ, Edgman‐Levitan S. Shared decision making‐‐pinnacle of patient‐centered care. N Engl J Med 2012; 366: 780‐781.
- 40. Melson EDM, Malhotra K, PCOS SEva working group, et al. A systematic review of models of care for polycystic ovary syndrome highlights the gap in the literature, especially in developing countries. Front Endocrinol (Lausanne) 2023; 14: 1217460.
- 41. Tay CT, Pirotta S, Teede HJ, et al. Polycystic ovary syndrome models of care: a review and qualitative evaluation of a guideline‐recommended integrated care. Semin Reprod Med 2021; 39: 133‐142.
- 42. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012; 27: 1361‐1367.
- 43. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod Update 2012; 18: 618‐637.
- 44. Awoke MA, Earnest A, Joham AE, et al. Weight gain and lifestyle factors in women with and without polycystic ovary syndrome. Hum Reprod 2021; 37: 129‐141.
- 45. Colombo GE, Dafauce BX, Patten RK, et al. Comparison of selected exercise training modalities in the management of PCOS: A systematic review and meta‐analysis to inform evidence‐based guidelines. JSAMS Plus 2023; 2: 100024.
- 46. Department of Health and Aging. Physical activity and sedentary behaviour guidelines for adults [website]. Canberra, Australia; Department of Health and Aging. 2014. https://www.health.gov.au/topics/physical‐activity‐and‐exercise/physical‐activity‐and‐exercise‐guidelines‐for‐all‐australians (viewed June 2024).
- 47. National Health and Medical Research Council. Australian Dietary Guidelines. Canberra, Australia: National Health and Medical Research Council; 2013. https://www.nhmrc.gov.au/adg (viewed June 2024).
- 48. Rosenzweig JL, Bakris GL, Berglund LF, et al. Primary prevention of ASCVD and T2DM in patients at metabolic risk: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab 2019; 104: 3939‐3985.
- 49. Tylka TL, Annunziato RA, Burgard D, et al. The weight‐inclusive versus weight‐normative approach to health: evaluating the evidence for prioritizing well‐being over weight loss. J Obes 2014; 983495.
- 50. Forslund M, Melin J, Alesi S, et al. Combined oral contraceptive pill compared with no medical treatment in the management of polycystic ovary syndrome: a systematic review. Clin Endocrinol (Oxf) 2023; 99: 79‐91.
- 51. Forslund M, Melin J, Alesi S, et al. Different kinds of oral contraceptive pills in polycystic ovary syndrome: a systematic review and meta‐analysis. Eur J Endocrinol 2023; 189: S1‐S16.
- 52. Melin J, Forslund M, Alesi S, et al. Metformin and combined oral contraceptive pills in the management of polycystic ovary syndrome: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2023: 109: e817‐836.
- 53. World Health Organization Department of Sexual and Reproductive Health and Research and Johns Hopkins Bloomberg School of Public Health, Center for Communication Programs (CCP), Knowledge SUCCESS. Family planning: a global handbook for providers (2022 update). Geneva, Switzerland; Baltimore, United States of America: CCP and WHO; 2022. https://www.who.int/publications/i/item/9780999203705 (viewed June 2024).
- 54. Alesi S, Forslund M, Melin J, et al. Efficacy and safety of anti‐androgens in the management of polycystic ovary syndrome: a systematic review and meta‐analysis of randomised controlled trials. EClinicalMedicine 2023; 63: 102162.
- 55. Melin J, Forslund M, Alesi S, et al. The impact of metformin with or without lifestyle modification versus placebo on polycystic ovary syndrome: a systematic review and meta‐analysis of randomized controlled trials. Eur J Endocrinol 2023; 189: S37‐S63.
- 56. Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis 2022; 18: 1345‐1356.






Abstract
Introduction: The Australian‐led2023 International evidence‐based guideline for the assessment and management of polycystic ovary syndrome was based on best available evidence, clinical expertise and consumer preference. It followed best practice, involved extensive evidence synthesis and applied relevant frameworks across evidence quality, feasibility, acceptability, cost and implementation. Thirty‐nine societies and organisations covering 71 countries were engaged. The evidence in the assessment and management of polycystic ovary syndrome (PCOS) has generally improved in the past five years, but remains of low to moderate quality. The technical evidence report, 52 systematic reviews and analyses (approximately 6000 pages) underpin 77 evidence‐based and 54 consensus recommendations, with 123 practice points.
Main recommendations: Changes include:
Changes in management as a result of this guideline: The 2023 guideline is approved by the National Health and Medical Research Council and provides clinicians and patients with clear advice on best practice in a common and neglected condition, based on the best available evidence, expert multidisciplinary input and consumer preferences. It provides vital, extensive patient and provider resources to enhance evidence‐based care.
The full guideline is available at www.monash.edu/medicine/mchri/pcos/guideline.