Thousands of bluebottle (Physalia sp.) stings occur each year in Australia.1 Stings cause immediate, intense pain that usually resolves within an hour and is associated with a characteristic linear erythematous eruption (Box 1).2 The first aid management of bluebottle stings is a daily problem for surf lifesavers. Currently, most first aid bodies, including the International Life Saving Federation,3 recommend treatment by topical application of ice packs. There is little scientific evidence to support this,2 and the only study to investigate ice packs was observational with no objective measure of pain, or control or comparator treatment.4
Many marine venoms are heat-labile in vitro.5-8 It is feasible that heat penetrates the human dermis to the estimated depth that nematocysts inject toxins (100–1000 μm),1 and recent clinical research suggests heat may be effective for treating jellyfish stings.9,10 However, previous studies of heat therapy were small10,11 or not randomised,11,12 and only one was published in full.12 A randomised controlled trial showed that, compared with ice packs, hot showers significantly reduced pain and treatment duration for bluebottle stings.10
If heat is to be used, it needs to be applied continuously. Furthermore, it has to be easily and rapidly administered at the beach. The risk of burns is temperature- and time-dependent, and superficial burns have been reported only with skin exposures longer than 1 hour and with temperatures over 46°C.13 Therefore, we chose immersion of the sting site in 45°C water for 20 minutes as an appropriate safe treatment for bluebottle stings, and compared this with the currently recommended treatment using ice packs.
Subjects presenting for treatment of an apparent bluebottle sting were eligible for the study. They were included if they had immediate localised pain and observed a bluebottle, or they had the characteristic linear wheal and flare reaction. Children under the age of 8 years were ineligible as the visual analogue scale (VAS: the tool we used for assessing the primary outcome measure) is not validated for this age group.15 Patients were also excluded if they had a sting to the eye or appeared sufficiently unwell that ambulance attention was required.
Adhesive tape (3M, St Paul, Minn, USA) was placed over the sting site and then stuck to a numbered microscope slide.16 Nematocysts were identified microscopically by one author (J E S) using cnidome libraries.
The primary outcome for the study was a clinically important reduction in pain 10 and 20 minutes after treatment. A clinically important reduction in pain was taken to be the reduction in the VAS score equivalent to the patient description of “a lot better” (Box 2).17
Secondary outcomes were the development of regional or radiating pain, generalised pain, frequency of systemic symptoms (nausea, vomiting, respiratory symptoms), crossover to the alternative treatment, and proportion with pruritus or rash on follow-up. For secondary analyses, patients were only included if data for the outcome were available. All primary and secondary outcomes were decided a priori and registered with the ethics committee.
The sample size was based on the assumption that a reduction in pain would be reported by 30% of patients after ice treatment and 48% after heat treatment.10 Our study was powered at 80% to detect a 20% absolute increase in patients with clinically important pain reduction after hot water immersion compared with ice packs, at an α level of 0.05. The number of patients required was 190.
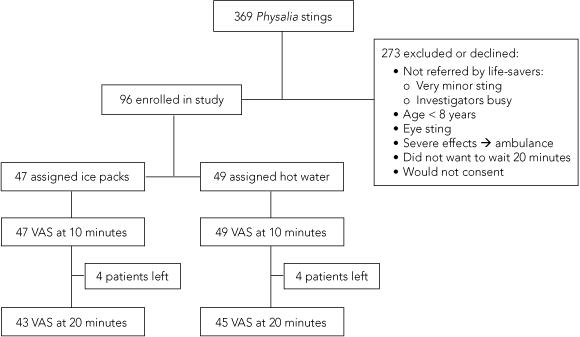
Ninety-six patients were enrolled, of a possible 369 people in the study (Box 3). Forty-nine were treated with hot water and 47 with ice packs. All randomly assigned patients underwent their designated treatment and completed a VAS at 10 minutes, but eight patients did not remain for the 20 minutes (Box 3). The two groups had similar baseline features, except patients treated with hot water had more severe initial pain than those treated with ice packs (Box 4).
After 10 minutes, 26 (53%) of the hot water group had clinically reduced pain versus 15 (32%) treated with ice packs (Box 5). After 20 minutes, 39 (87%) of the hot water group had clinically reduced pain versus 14 (33%) treated with ice packs. Box 6 shows all patients divided into their treatment groups and whether they had a clinically important pain reduction. All patients treated with hot water who had less pain at 10 minutes remained better at 20 minutes, but three patients treated with ice packs who had less pain at 10 minutes reported worsening pain at 20 minutes.
Box 5 also shows results for patients with nematocyst-confirmed stings, and for patients for whom the initial VAS was matched to within 2 mm.
Radiating pain occurred less with hot water (difference, −20%; 95% CI, −39% to −0.003%; P = 0.039), and systemic effects were uncommon in both groups (Box 7). Respiratory symptoms were reported in one patient, who had chest tightness that resolved spontaneously.
The proportion of patients with itch, redness or rash at 24 hours was similar in both groups (Box 7). Two patients (one in each group) developed bullae within 48 hours of the sting; these took 1 to 2 weeks to resolve.
We found that hot water immersion was highly effective compared with ice packs in the treatment of pain caused by bluebottle stings. The treatment effect with hot water immersion improved between 10 and 20 minutes (53% to 87% with clinically important pain reduction), whereas there was no further improvement (from 32%) for patients treated with ice packs. This was similar to a previous study, in which 30% responded to ice packs, and suggests that the response to ice packs may have been a placebo effect.18,19
The measurement of pain is problematic because it is subjective and is influenced by numerous factors. However, pain is the most important and distressing effect of bluebottle stings, so it was essential that we establish the effect of treatment on pain. The VAS has become a standard tool for the measurement of pain in research, and has been validated in numerous settings.17 Recently, it has been used in studies of painful envenoming, including jellyfish stings and widow spider bites.9,10,20 Some controversy exists over what constitutes a clinically important reduction in pain and whether this amount varies depending on baseline score. Studies measuring acute pain have compared the numerical change in the VAS with the patient’s description of a “lot less pain”.17,21,22 In this study, we chose to define a clinically important reduction in pain as a “lot less” according to the criteria of Bird and Dickson,17 where a clinically important reduction in pain varied with initial VAS (Box 2).
Another limitation was the possible presence of allocation bias, suggested by the baseline imbalance in pain severity. Patients gave consent to participate before being randomly allocated a treatment and were accepted in order of presentation. However, bluebottles occur in clusters, so there were sometimes large numbers of potential recruits with stings of varying severity. We suspect in some cases when two or three patients were simultaneously recruited (often one parent consenting for multiple children), the research assistants may have allocated hot water treatment to the more severe stings once the envelopes were open. However, this was likely to be rare, and a post-hoc analysis using simulations of matched treatment subgroups still showed a highly significant outcome at 20 minutes (Box 6).
Our study demonstrated a greater improvement with hot water treatment than Bowra et al reported (87% versus 48%).10 This is probably because the immersion technique maintained a constant 45°C temperature directly in contact with the sting site. Showers (as used by Bowra et al10) deliver variable water temperature depending on height, nozzle design and settings on the water mixer. The immersion technique was also safer because it prevented exposure to temperatures over 46°C.13 The use of thermostatic mixing valves meant it was possible to supply water at exactly 45°C. In our experience, the technique was cost-effective and practical at beach first aid stations once thermostatic mixing valves had been installed.
It might be argued that the hot water immersion may be a symptomatic treatment for jellyfish stings, rather than providing definitive treatment by inactivating venom. A similar controversy exists for venomous fish stings, because hot water immersion has been recommended for decades on the basis that fish venoms are heat labile.5 However, it is a common observation that the pain of venomous fish stings is only alleviated while the injured part is immersed, suggesting that venom is not inactivated and treatment is only symptomatic.2 In jellyfish stings, the venom remains close to the surface, unlike penetrating fish injuries where venom is injected deeper.1,2 We demonstrated a time-dependent effect of hot water immersion, with a barely significant effect at 10 minutes and a highly significant effect at 20 minutes. In addition, pain did not recur. This leads us to suggest that the mechanism of reducing pain by heat treatment is inactivation of venom.
2 Definition of clinically important reduction in pain17

A clinically important reduction in pain was taken to be the reduction in the visual analogue scale (VAS) score equivalent to the patient description of “a lot better”, as defined by Bird and Dickson.17 This change in millimetres on the VAS is dependent on the baseline starting point and is 16 mm for an initial VAS in the range 0–33 mm, 33 mm for 34–66 mm and 48 mm for 67–100 mm. The figure shows a VAS divided into three and the associated reduction in VAS required to indicate a clinically important reduction in pain.
3 Recruitment and treatment of patients presenting with an apparent bluebottle sting at two beaches in Newcastle between 30 December 2003 and 5 March 2005
4 Baseline characteristics of the two treatment groups
* Age was not known for two patients receiving hot water and one receiving ice packs. |
|||||||||||||||
6 Change (reduction) in visual analogue scale (VAS) at 20 minutes (VAS20 – VAS0) compared with baseline (VAS0) for all patients

Patients receiving hot water and patients receiving ice packs are divided into three groups based on their initial VAS (see Box 2). The black lines divide patients who had clinically important reduction in pain (above the line) from those who did not (below the line).
Received 18 December 2005, accepted 13 February 2006
- Conrad Loten1
- Barrie Stokes2
- David Worsley1
- Jamie E Seymour3
- Simon Jiang2
- Geoffrey K Isbister4,5
- 1 Hunter New England Area Health Service, Newcastle, NSW.
- 2 University of Newcastle, Newcastle, NSW.
- 3 Department of Tropical Biology, James Cook University, Cairns, QLD.
- 4 Tropical Toxinology Unit, Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 5 Department of Clinical Toxicology, Newcastle Mater Hospital, Newcastle, NSW.
We thank all the members of the surf lifesaving clubs and surf lifesavers employed by Newcastle City Council for their assistance in identifying patients for the study. We particularly thank Nobby’s and Dixon Park Surf Lifesaving Clubs for allowing us to use space in their clubs and install the thermostatic mixing valves. We thank Enware Australia for providing cost-price valves and Macs Plumbing for their installation. We thank John Bolton for assistance in obtaining council permission to undertake the study and Kate Armstrong and Paul Healey for recruiting patients. We thank Tony Smith for his comments on the manuscript. The study was partially funded by a Margaret Mitchell Grant (Newcastle Mater Hospital Internal Grant) and a donation from the NSW Surf Life Saving Council. Geoffrey Isbister is supported by a National Health and Medical Research Council Clinical Career Development Award ID300785.
None identified.
- 1. Williamson JA, Fenner PJ, Burnett JW, Rifkin JF. Venomous and poisonous marine animals. 1st ed. Sydney: UNSW Press, 1996.
- 2. Isbister GK. Marine envenomation and poisoning. In: Dart RC, editor. Medical toxicology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2004: 1621-1644.
- 3. Fenner P. International Life Saving Federation policy statement 6: marine envenomation. Belgium: International Life Saving Federation, 2000: 1-5.
- 4. Exton DR, Fenner PJ, Williamson JA. Cold packs: effective topical analgesia in the treatment of painful stings by Physalia and other jellyfish. Med J Aust 1989; 151: 625-626.
- 5. Church JE, Hodgson WC. The pharmacological activity of fish venoms. Toxicon 2002; 40: 1083-1093.
- 6. Bucherl W, Buckley EE. Venomous animals and their venoms. Vol 3. London: Academic Press, 1971.
- 7. Endean R. Separation of two myotoxins from nematocysts of the box jellyfish (Chironex fleckeri). Toxicon 1987; 25: 483-492.
- 8. Carrette TJ, Cullen P, Little M, et al. Temperature effects on box jellyfish venom: a possible treatment for envenomed patients? Med J Aust 2002; 177: 654-655. <MJA full text>
- 9. Nomura JT, Sato RL, Ahern RM, et al. A randomized paired comparison trial of cutaneous treatments for acute jellyfish (Carybdea alata) stings. Am J Emerg Med 2002; 20: 624-626.
- 10. Bowra J, Gillett M, Morgan J, Swinburn E. Randomised crossover trial comparing hot showers and icepacks in the treatment of Physalia envenomation [abstract]. Emerg Med 2002; 14: A22.
- 11. Lopez EA, Weisman RS, Bernstein J. A prospective study of the acute therapy of jellyfish envenomations [abstract]. J Toxicol Clin Toxicol 2000; 38: 512.
- 12. Thomas CS, Scott SA, Galanis DJ, Goto RS. Box jellyfish (Carybdea alata) in Waikiki: their influx cycle plus the analgesic effect of hot and cold packs on their stings to swimmers at the beach: a randomized, placebo-controlled, clinical trial. Hawaii Med J 2001; 60: 100-107.
- 13. Moritz AR, Henriques FC. Studies in thermal injury II: the relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol 1947; 23: 695-720.
- 14. O’Reilly GM, Isbister GK, Lawrie PM, et al. Prospective study of jellyfish stings from tropical Australia, including the major box jellyfish Chironex fleckeri. Med J Aust 2001; 175: 652-655. <MJA full text>
- 15. Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001; 37: 28-31.
- 16. Currie BJ, Wood YK. Identification of Chironex fleckeri envenomation by nematocyst recovery from skin. Med J Aust 1995; 162: 478-480.
- 17. Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med 2001; 38: 639-643.
- 18. Beecher HK. The powerful placebo. JAMA 1955; 159: 1602-1606.
- 19. Wampold BE, Minami T, Tierney SC, et al. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. J Clin Psychol 2005; 61: 835-854.
- 20. Ellis RM, Sprivulis PC, Jelinek GA, et al. A double-blind, randomized trial of intravenous versus intramuscular antivenom for red-back spider envenoming. Emerg Med Australas 2005; 17: 152-156.
- 21. Holdgate A, Asha S, Craig J, Thompson J. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg Med (Fremantle) 2003; 15: 441-446.
- 22. Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996; 27: 485-489.






Abstract
Objective: To investigate the effectiveness of hot water immersion for the treatment of Physalia sp. (bluebottle or Portuguese Man-of-War) stings.
Design: Open-label, randomised comparison trial. Primary analysis was by intention to treat, with secondary analysis of nematocyst-confirmed stings. One halfway interim analysis was planned.
Setting: Surf lifesaving first aid facilities at two beaches in eastern Australia from 30 December 2003 to 5 March 2005.
Participants: 96 subjects presenting after swimming in the ocean for treatment of an apparent sting by a bluebottle.
Interventions: Hot water immersion (45°C) of the affected part versus ice pack application.
Main outcome measures: The primary outcome was a clinically important reduction in pain as measured by the visual analogue scale (VAS). Secondary outcomes were the development of regional or radiating pain, frequency of systemic symptoms, and proportion with pruritus or rash on follow-up.
Results: 49 patients received hot water immersion and 47 received ice packs. The two groups had similar baseline features, except patients treated with hot water had more severe initial pain (VAS [mean ± SD]: 54 ± 22 mm versus 42 ± 22 mm). After 10 minutes, 53% of the hot water group reported less pain versus 32% treated with ice (21%; 95% CI, 1%–39%; P = 0.039). After 20 minutes, 87% of the hot water group reported less pain versus 33% treated with ice (54%; 95% CI, 35%–69%; P = 0.002). The trial was stopped after the halfway interim analysis because hot water immersion was shown to be effective (P = 0.002). Hot water was more effective at 20 minutes in nematocyst-confirmed stings (95% versus 29%; P = 0.002). Radiating pain occurred less with hot water (10% versus 30%; P = 0.039). Systemic effects were uncommon in both groups.
Conclusions: Immersion in water at 45°C for 20 minutes is an effective and practical treatment for pain from bluebottle stings.