We report the first human case of babesiosis in Australia, thought to be locally acquired
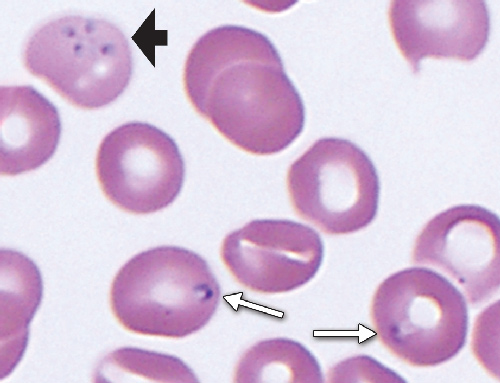
To identify the organism and further elucidate its source, blood samples and films from the patient were examined at the Centers for Disease Control and Prevention (CDC) in Atlanta, United States, and at the School of Veterinary and Biomedical Sciences, Murdoch University, Western Australia. Both laboratories agreed that the morphological characteristics of the parasites in the blood films were consistent with a small Babesia. Intraerythrocytic organisms were mostly single and measured 1.5–2.5 m in size but were highly polymorphic; pyriform and ovoid forms predominated, bizarre amoeboid forms were also common, and an occasional tetrad (Maltese cross form) was noted (Box 1). Immunofluorescent antibody testing for Babesia microti was performed by the CDC on serum samples from the patient (positive result, with titre of 1 : 256) and his son (negative result).
Complete sequencing of the 18S ribosomal RNA gene (18S rDNA) and partial sequencing of the β-tubulin gene (both amplified by polymerase chain reaction) confirmed that the organism in the patient’s blood was B. microti. At the CDC, a nested PCR that specifically amplifies a 154-base-pair fragment from the B. microti 18S rDNA was initially used to confirm the presence of B. microti in the patient’s blood. In addition, sequencing of a 1767-base-pair fragment amplified with primers Crypto FL (5'-AACCTGGTTGATCCTGCCAGTAGTCAT-3') and Crypto RN (5'-GAATGATCCTTCCGCAGGTTCACCTAC-3'), was performed to strengthen the PCR findings.1 The 18S rDNA sequence obtained was 100% similar to the GenBank entry AY693840 obtained from a B. microti isolate 18S rDNA gene. At Murdoch University, two nested sets of universal piroplasm 18S rDNA primers were used, one of which has been published.2 The patient’s son’s blood was used as a negative control. The consensus sequence was 100% homologous to known human-derived Babesia species isolates. In addition, five novel primer sets designed during this study were used to obtain a partial β-tubulin gene fragment (791 base pairs), which confirmed the presence of B. microti in the patient’s blood and showed 100% homology with North American isolates (eg, GenBank entries AB083377 and AY144722). A phylogenetic tree for this locus (produced using the maximum likelihood method) revealed clustering with isolates of B. microti obtained from the tick species Ixodes scapularis (formerly known as Ixodes dammini), humans and voles in North America.
Human babesiosis is an emerging tick-borne zoonosis. The first human case was reported in Croatia in 1957.3 In 1968, Babesia divergens was identified as the cause of human babesiosis in Europe; this was soon followed by the discovery of human cases of B. microti infection in the US.4 More recently, human cases of babesiosis have emerged from Asia, Africa and South America.5-8
Since B. microti has never been detected in Australia before, its discovery as the cause of infection in this patient, who had no significant history of travel, raises intriguing questions about its natural hosts and epidemiology on this continent. Traditionally, B. microti is considered to have a Holarctic distribution, associated with a variety of small mammalian hosts (rodents, including voles, and shrews), and is transmitted by several Ixodes tick species present throughout the northern hemisphere, with humans becoming infected as accidental hosts. Recent phylogenetic analyses based on complete sequences of the genes encoding 18S ribosomal RNA, β-tubulin and the η subunit of the chaperonin-containing t-complex polypeptide 1 suggest that B. microti represents a genetically diverse species complex that comprises several geographically distinct clusters located in North America, Eurasia and Japan, and is closely related to “Babesia microti-like” species isolated from an ever-expanding range of feral and domesticated mammal hosts.9
In Australia, babesiosis is a well documented disease of cattle (Babesia bigemina and Babesia bovis) and dogs (Babesia canis, Babesia vogeli and Babesia gibsoni), and babesiosis tick vectors have been imported to the continent since European settlement.10,11 Australia also has a diverse variety of native Ixodes ticks, including Ixodes holocyclus (responsible for tick paralysis), and a few Babesia and Theileria species have been described morphologically in native marsupial hosts (but not B. microti).12 Unfortunately, a paucity of molecular studies means that the taxonomy and phylogenetic relationships of the endemic piroplasms (intraerythrocytic tick parasites, including Babesia and Theileria) are not well understood.
The natural history of this patient’s infection is notable. Babesia ring forms were detectable on routine blood films taken while he was an outpatient — 7 months before he died. At that time, he was asymptomatic and neither anaemic nor thrombocytopenic, but 6 months later the babesiosis became symptomatic and severe. The parasitaemia of 5.1% around the time of his death was likely to have been an underestimate of the true figure as it would have been diluted by the multiple blood products that he was receiving at the time. Asymptomatic parasitaemia is well described in babesiosis, both in the setting of primary infection and following treatment of symptomatic infection.4 It is unclear what transformed this patient’s chronic asymptomatic infection into a severe symptomatic infection that probably contributed to his death. He did have risk factors for severe babesiosis: hyposplenism, liver impairment and his age;4 however, these were present when the infection was asymptomatic months earlier. It is possible that his chronic hospitalisation, and general deconditioning from the long admission, resulted in significant immunosuppression.
Severe babesiosis from B. microti is a serious condition with a case fatality rate of 5%–10%.13 Indeed, this patient’s condition did not improve despite his receiving recommended therapy once the diagnosis of babesiosis was made. Even the artesunate that he received for suspected malaria (immediately before the diagnosis) has been shown to have activity against B. microti in animal models.14
Although the animal host for B. microti is yet to be identified in Australia, the proximity of ticks, other wildlife and human populations along Australia’s eastern seaboard means that further cases may be encountered. Clinicians working in Australia should therefore be aware of the signs and symptoms of babesiosis and how to diagnose it (Box 2). Further investigation into the piroplasms of native mammals, introduced rodents and their ticks is necessary to identify the source of this infection. As transfusion-related babesiosis is well recognised in other countries,15 this case may have future implications for the screening of blood products in Australia.
1 Blood film from a 56-year-old man infected with Babesia microti, showing a tetrad form (black arrow) and single ovoid forms (white arrows)
- 1. Herwaldt BL, Cacciò S, Gherlinzoni F, et al. Molecular characterization of a non–Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis 2003; 9: 942-948.
- 2. Jefferies R, Ryan UM, Irwin PJ. PCR-RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet Parasitol 2007; 144: 20-27.
- 3. Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop 1957; 9: 11-16.
- 4. Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22: 469-488.
- 5. Kim JY, Cho SH, Joo HN, et al. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol 2007; 45: 2084–2087.
- 6. Bush JB, Isaäcson M, Mohamed AS, et al. Human babesiosis–a preliminary report of 2 suspected cases in South Africa. S Afr Med J 1990; 78: 699.
- 7. Rios L, Alvarez G, Blair S. Serological and parasitological study and report of the first case of human babesiosis in Colombia. Rev Soc Bras Med Trop 2003; 36: 493-498.
- 8. Marathe A, Tripathi J, Handa V, Date V. Human babesiosis–a case report. Indian J Med Microbiol 2005; 23: 267-269.
- 9. Nakajima R, Tsuji M, Zamoto-Niikura A, et al. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J Vet Med Sci 2009; 71: 55-68.
- 10. Bock R, Jackson L, de Vos A, Jorgensen W. Babesiosis of cattle. Parasitology 2004; 129 Suppl: S247-S269.
- 11. Jefferies R, Ryan UM, Jardine J, et al. Blood, bull terriers and babesiosis: further evidence for direct transmission of Babesia gibsoni in dogs. Aust Vet J 2007; 85: 459-463.
- 12. Clark P. Haematology of Australian mammals. 1st ed. Melbourne: CSIRO Publishing, 2004.
- 13. Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001; 32: 1117-1125.
- 14. Goo YK, Terkawi MA, Jia H, et al. Artesunate, a potential drug for treatment of Babesia infection. Parasitol Int 2010; 59: 481-486.
- 15. Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev 2011; 24: 14-28.





We thank Michael Pidcock, Grace Moshi and Rachel Murray (Canberra Hospital) for their haematological expertise and Bert de Vos (Biosecurity Queensland) for his advice on bovine babesiosis.
No relevant disclosures.