We have previously described a statistically significant and increasing excess in prostate cancer mortality among men living in regional and rural areas of Australia compared with those in capital cities.1 Our data indicated that the probability of having a prostate-specific antigen (PSA) test or management of prostate cancer by radical prostatectomy, as well as the likelihood of survival, depended on where a man lived.1
Because of the increasing focus on health inequalities experienced by Australians living in rural areas,2 and the possibility that reported temporal trends might change over a longer study period,3,4 we have collected more recent data and conducted a similar analysis of trends to assess whether urban–rural differences in PSA testing, prostate cancer incidence, radical prostatectomy and prostate cancer mortality still exist. Additionally, we have investigated whether there is an urban–rural difference in relative survival rates among men diagnosed with prostate cancer and in PSA testing specifically for screening purposes.
We used similar methods to our previous study,1 limiting our analysis to men aged 50–79 years. Time periods, codes for and sources of data for each outcome measure are shown in Box 1. A Medicare item number specifically for PSA screening was fully introduced in the 2001–02 financial year. Geographical location was based on subjects’ usual address, categorised according to Statistical Divisions into “capital city” versus the rest of Australia (“regional and rural areas”).5
We calculated directly age-standardised annual rates6 per 100 000 men aged 50–79 years, stratified by geographical location, for each of the outcome measures. We also computed the ratios of the age-standardised rates for men from regional and rural areas (“rural”) compared with men living in capital cities (“urban”) each year for each outcome measure. Joinpoint regression was used to determine the associated trend lines (Joinpoint Regression Program, Version 3.0, 2005, US National Cancer Institute, Bethesda, Md, USA).
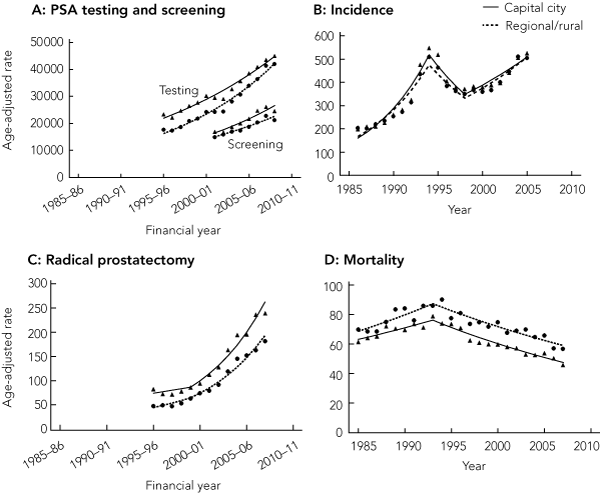
The rate of PSA testing increased in capital cities and regional and rural areas (Box 2), although the converging trends mean that the rural : urban rate ratio for PSA testing has moved significantly closer to unity over time (from 0.76 [95% CI, 0.75–0.76] in 1995–96 to 0.93 [95% CI, 0.93–0.94] in 2008–09).
The rate of PSA testing carried out specifically for screening purposes likewise increased in both urban and rural areas (Box 2). In 2008–09, the PSA screening rate in rural areas was 21 267 per 100 000 men aged 50–79 years, and in urban areas was 24 606 per 100 000 (P < 0.01). In contrast to the rates for all PSA testing, the rural : urban rate ratio for PSA screening has shown a small, non-significant downward shift away from unity over time (Box 3).
Because of similar prostate cancer incidence trends for capital city residents and regional and rural residents over time (Box 2), the rural : urban incidence rate ratio has tended to fluctuate around or slightly below unity for most of the period studied (Box 3).
There was a sharp increase in the age-standardised rate of radical prostatectomy procedures from 1999–00 onwards for urban and rural men (Box 2). However, men living in regional and rural areas remained significantly less likely to have had a radical prostatectomy compared with their capital city counterparts (2007–08: rural, 182.2/100 000 men; urban, 239.2/100 000 men; P < 0.01).
Nationally, 5-year relative survival among men diagnosed with prostate cancer increased from 61.3% (95% CI, 60.5%–62.1%) during the 1980s to 83.7% (95% CI, 83.3%–84.1%) in the 1990s and 89.9% (95% CI, 89.4%–90.4%) during the early 2000s. While these increases were evident among men living in both urban and rural areas, survival was lower among rural men, with this differential widening over time (5-year relative survival, 2000–2004: regional/rural, 87.7%; urban, 91.4%; P < 0.01) (Box 4).
A decreasing trend in prostate cancer mortality that commenced in 1993 for men living in both urban and rural areas continued until 2007 (Box 2). Overall, prostate cancer mortality among men in rural areas remained significantly higher than in urban areas. In 2007, the mortality rate was 56.9 per 100 000 men in rural areas compared with 45.8 per 100 000 men in urban areas (P < 0.01) (Box 2). The rural : urban rate ratio showed an increasing trend over the study period, rising to 1.24 (95% CI, 1.11–1.38) in 2007 (Box 3).
Our updated analysis shows overall increases in rates of PSA screening and radical prostatectomy, reductions in mortality and improvements in survival throughout Australia. Although incidence rates for prostate cancer in both urban and rural parts of Australia are similar, the most recent data available show that the excess in prostate cancer mortality for men living in regional and rural areas has continued to increase. This is consistent with the excess in mortality from all causes experienced by men living in rural areas of the country.7 Further, rates of radical prostatectomy and PSA screening continue to be lower among rural men, and although survival rates for prostate cancer in Australia are among the highest in the world,8 survival outcomes remain poorer among rural men than urban men.
The distinctive prostate cancer incidence rate trends over time have been strongly influenced by the uptake of PSA screening.4 In isolation, similar urban and rural incidence trends could suggest that PSA screening has had an equivalent impact on the diagnosis of prostate cancer in urban and rural areas. This hypothesis is countered by the consistently lower rates of PSA screening in rural areas demonstrated in this study. Similar incidence trends, lower rates of PSA screening and lower rates of radical prostatectomy (a procedure specific to the treatment of localised prostate cancer) would, however, be consistent with the hypothesis that a greater proportion of men diagnosed with prostate cancer in rural areas of Australia are diagnosed because they show symptoms. This had been reported previously in New South Wales9 and is reinforced by the difference between areas in age at diagnosis, as shown in this study.
Problematically, data on cancer stage or even serum PSA level at diagnosis are not available on a national basis. In NSW, poorer survival outcomes9 and lower rates of radical prostatectomy10 have been shown among rural men. While stage was an important factor, these differentials still remained after adjusting for spread of disease at diagnosis,9,10 suggesting there may be other unknown factors contributing to the survival differential.
Even in an environment of equitable PSA screening, the extent to which prostate cancer patients have access to different treatment options and follow-up care is unknown. For example, in the absence of clear national guidelines surrounding the results of PSA screening, systematic variations in practice may occur. As well, in regional areas where urological and radiation services are sparser, patterns of care will vary. Further research is required to quantify the associations between prostate cancer diagnostic and treatment outcomes and key area-level characteristics and individual-level demographic, clinical and psychosocial factors,11 so that health services policy and planning strategies to manage this disease can be guided by evidence.
Of the variables included in this study, PSA screening is the most amenable to change. Recommending an increase in the rate of PSA screening in rural areas is currently problematic because of the equivocal and controversial evidence for PSA screening. Since our earlier report,1 initial results of two large-scale prostate cancer screening trials have been published.12,13 Although results were analysed at earlier follow-up time points than is optimal for assessing prostate cancer survival benefit, these international studies suggest that in the presence of already high PSA testing rates, organised PSA population screening programs are likely to result in overdiagnosis and overtreatment with little reduction in mortality.14 Conversely, where the prevalence of asymptomatic detection is low, there is some evidence of greater potential for screening programs to save lives.15
It remains unclear whether raising the prevalence of PSA screening would reduce the inequity in outcomes among rural men in Australia, given that our data show that a relatively high level of screening (21% of rural compared with 25% of urban men) already occurs. Any negatives in terms of overdiagnosis and overtreatment need to be weighed against lower rates of surgical treatment, poorer survival and higher mortality due to prostate cancer in regional and rural parts of the country. It has been suggested that, under these circumstances, a well coordinated screening program, in conjunction with improved access to specialised diagnostic, monitoring and treatment services, may reduce the incidence of advanced prostate cancer.14
Due to the difficulties in obtaining a consistent geographical concordance across time, we were intentionally conservative in defining urban areas. It is likely that the “rural” group contains a substantial proportion of men living on the outskirts of the capital city boundaries and experiencing similar access to diagnostic and treatment services as those men living in capital cities. Thus our results could reflect an underestimate of the true rural–urban differential. That we were unable to obtain population-based data on the use of radiotherapy procedures is also a limitation, since this is the other major form of treatment with curative intent for men with prostate cancer.14 Finally, it remains possible that the lack of change in the mortality differential is influenced by the typically longer time interval between prostate cancer diagnosis and death.16 However, since the widespread introduction of PSA testing in Australia in 1993, this explanation is becoming increasingly less likely.
1 Time periods, codes for and sources of data for each outcome measure reported
Australian Cancer Database, Australian Institute of Health and Welfare |
|||||||||||||||
Australian Cancer Database, Australian Institute of Health and Welfare |
|||||||||||||||
2 Age-standardised rates per 100 000 men* for PSA testing and screening, prostate cancer incidence, radical prostatectomy and prostate cancer mortality
PSA = prostate-specific antigen.
* Age-standardised to 2001 Australian Standard Population.6 Trends are modelled using joinpoint regression. Linear trends (below) are expressed in terms of estimated average yearly percentage change.
3 Rate ratios* for men living in regional and rural areas compared with men living in capital cities for PSA screening, prostate cancer incidence, radical prostatectomy and prostate cancer mortality
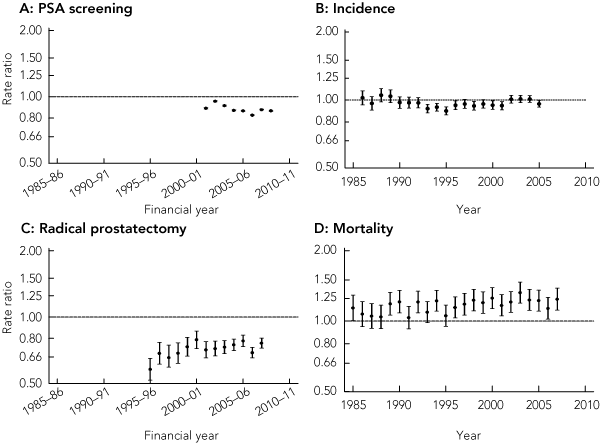
A: Trend for PSA screening rate ratios: 2001–02 to 2008–09, − 1.0% (95% CI, − 2.4% to +0.3%).
C: Trend for radical prostatectomy rate ratios: 1995–96 to 2007–08, +1.0% (95% CI, − 0.0 to +2.1%).
D: Trend for prostate cancer incidence rate ratios: 1985–2007, +0.6% (95% CI, +0.2% to +1.0%).
Received 1 November 2010, accepted 16 January 2011
- Peter D Baade1,2
- Danny R Youlden1
- Michael D Coory3
- Robert A Gardiner4
- Suzanne K Chambers5,1
- 1 Viertel Centre for Research in Cancer Control, Cancer Council Queensland, Brisbane, QLD.
- 2 School of Public Health, Queensland University of Technology, Brisbane, QLD.
- 3 Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, VIC.
- 4 University of Queensland Centre for Clinical Research, Royal Brisbane Hospital, Brisbane, QLD.
- 5 Griffith Health Institute, Griffith University, Gold Coast, QLD.
We acknowledge the assistance of staff from the Cancer and Screening Unit of the Australian Institute of Health and Welfare who extracted the required data from the Australian Cancer Database.
Robert Gardiner has received payment from sanofi-aventis as a consultant for a manuscript on benign prostatic hyperplasia.
- 1. Coory MD, Baade PD. Urban–rural differences in prostate cancer mortality, radical prostatectomy and prostate-specific antigen testing in Australia. Med J Aust 2005; 182: 112-115.
- 2. Armstrong BK, Gillespie JA, Leeder SR, et al. Challenges in health and health care for Australia. Med J Aust 2007; 187: 485-489.
- 3. Baade PD, Coory MD, Aitken JF. International trends in prostate-cancer mortality: the decrease is continuing and spreading. Cancer Causes Control 2004; 15: 237-241.
- 4. Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009; 53: 171-184.
- 5. Australian Bureau of Statistics. Statistical geography Volume 1 – Australian Standard Geographical Classification (ASGC). Canberra: ABS, 2006. (ABS Cat. No. 1216.0.)
- 6. Australian Bureau of Statistics. Population by age and sex – Australian states and territories. Canberra: ABS, 2003. (ABS Cat. No. 3201.0.)
- 7. Australian Institute of Health and Welfare. Rural, regional and remote health: indicators of health status and determinants of health. (AIHW Cat. No. PHE 97; Rural Health Series No. 9.) Canberra: AIHW, 2008.
- 8. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008; 9: 730-756.
- 9. Jong KE, Smith DP, Yu XQ, et al. Remoteness of residence and survival from cancer in New South Wales. Med J Aust 2004; 180: 618-622.
- 10. Hayen A, Smith DP, Patel MI, et al. Patterns of surgical care for prostate cancer in NSW, 1993-2002: rural/urban and socio-economic variation. Aust N Z J Public Health 2008; 32: 417-420.
- 11. Baade PD, Aitken JF, Ferguson M, et al. Diagnostic and treatment pathways for men with prostate cancer in Queensland: investigating spatial and demographic inequalities. BMC Cancer 2010; 10: 452.
- 12. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360: 1310-1319.
- 13. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360: 1320-1328.
- 14. Denham JW, Bender R, Paradice WE. It’s time to depolarise the unhelpful PSA-testing debate and put into practice lessons from the two major international screening trials. Med J Aust 2010; 192: 393-396.
- 15. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010; 11: 725-732.
- 16. Etzioni R, Legler JM, Feuer EJ, et al. Cancer surveillance series: interpreting trends in prostate cancer. Part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst 1999; 91: 1033-1039.





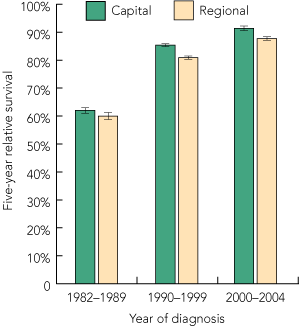
Abstract
Objective: To update our previous analysis of trends for prostate-specific antigen (PSA) testing, prostate cancer incidence, radical prostatectomy and prostate cancer mortality to assess whether men in rural and regional areas of Australia now have more equitable access to prostate cancer services, and improved outcomes.
Design, setting and participants: Descriptive study using population-based data for Australian men aged 50–79 years from 1982 to the 2008–09 financial year (depending on data availability for each outcome measure).
Main outcome measures: Age-standardised rates per 100 000 men and 5-year survival rates.
Results: Overall, rates of PSA screening and radical prostatectomy increased, accompanied by reductions in mortality and improvements in survival throughout Australia. Incidence rates were similar for men in urban and rural areas. However, in the last year of data collection, for men in rural areas compared with urban areas, rates of PSA screening (21 267/100 000 v 24 606/100 000; P < 0.01) and radical prostatectomy (182.2/100 000 v 239.2/100 000; P < 0.01) remained lower, mortality remained higher (56.9/100 000 v 45.8/100 000; P < 0.01), and survival outcomes continued to be poorer (5-year relative survival, 87.7% v 91.4%; P < 0.01).
Conclusions: With some limitations, these ecological data demonstrate that the use of diagnostic and treatment services among men living in rural areas of Australia remains lower than among their urban counterparts, their survival and mortality outcomes are poorer, and these differentials are continuing. There is an urgent need to explore further the reasons for these differences and to implement changes so these inequalities can be reduced.