Australian Aboriginal children are prone to frequent and severe episodes of acute otitis media (AOM). In a 2003 survey of over 600 children living in 29 Aboriginal communities throughout the Northern Territory, 20% had tympanic membrane perforation, despite 87% uptake of pneumococcal conjugate vaccine.1 Such high rates of AOM with perforation (AOMwiP) and chronic suppurative otitis media (CSOM) are seldom reported in the medical literature. A contributing factor to disease progression may be failed detection of AOM due to the absence of acute symptoms (pain, irritability or fever) in the presence of a bulging tympanic membrane.2 Thus, a diagnosis of AOM without perforation (AOMwoP) is recommended if a bulging tympanic membrane is present, whether symptoms are present or not.3 There are few studies assessing antibiotic treatment in such high-risk populations.
We have found that, compared with placebo, long-term antibiotic treatment resolves middle ear effusion and reduces perforation.4 However, these regimens are difficult to deliver effectively,5 particularly to poor families in remote settings. Previous studies have shown that short-course azithromycin (for 3–5 days),6-9 weekly single-dose azithromycin prophylaxis for 12 weeks10 or a single dose of azithromycin11,12 are effective for treating AOM and are less likely than amoxycillin clavulanate to be associated with gastrointestinal side effects.8
The primary aim of our study was to determine the clinical effectiveness of a single dose of azithromycin compared with a standard 7-day treatment course of amoxycillin13 in a population at high risk of tympanic membrane perforation and CSOM.
We categorised middle ear states into one of six diagnostic categories, using criteria based on recommendations for clinical practice in this population:3
-
Normal;
-
Otitis media with effusion (intact and non-bulging tympanic membrane and type B tympanogram);
-
AOMwoP (any bulging of the tympanic membrane and type B tympanogram);
-
AOMwiP (middle ear discharge observed and perforation recently healed or present for less than 6 weeks or covering less than 2% of the pars tensa of the tympanic membrane [CSOM is nearly always associated with larger perforations]);
-
Dry perforation (tympanic membrane perforation without any discharge observed); or
-
CSOM (middle ear discharge observed and perforation present for longer than 6 weeks and covering at least 2% of the pars tensa of the tympanic membrane).
The final middle ear diagnosis reflected the child’s more severely affected ear (highest category).
The presence of nasal discharge was recorded if nasal discharge was visible from 1 metre away at any time during the clinical examination. Skin sores (raised crust or pus visible) were recorded if visible on the arms, legs or head.
Nasal swabs were taken at baseline and at follow-up examinations. Swabs of ear discharge were also collected on the days when nasal swabs were taken. All swabs were collected, transported, stored and cultured as previously described.14 Azithromycin resistance was defined as a minimum inhibitory concentration (MIC) of ≥ 2 µg/mL. High-level resistance was defined as MIC > 32 µg/mL.
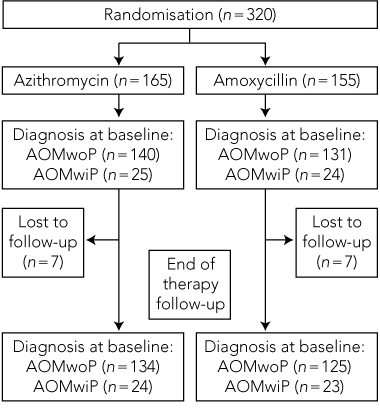
Clinical observations. Of 320 children taking part in the trial, 165 were randomly allocated to the azithromycin treatment group and 155 to the amoxycillin treatment group. As seven children were lost to follow-up in each group, analysis was carried out on the remaining 306 children (Box 2). Most were less than 2 years of age, and about half were male. For each group at baseline, 85% had AOMwoP and 15% had AOMwiP, and 5% of mothers reported that they thought their child had ear pain. Nasal discharge was seen in 69% of children and skin sores in 6%–7%.
Nasal carriage. For both treatment groups, nasal carriage of S. pneumoniae and NCHi was 83% or more. About 14% of children were colonised by S. pneumoniae with intermediate or full resistance to penicillin (MIC ≥ 0.12 µg/mL), and about 5% carried azithromycin-resistant S. pneumoniae (MIC ≥ 2 µg/mL). Less than 10% of children swabbed carried β-lactamase-producing NCHi.
Ear discharge. In the azithromycin and amoxycillin groups, respectively, S. pneumoniae was cultured from 22% and 32% of ear discharge swabs, and NCHi from 36% and 41% of ear discharge swabs.
Clinical failure (Box 3). At the end of therapy, 50% of the azithromycin group and 54% of the amoxycillin group were clinical failures. Similar proportions (45% and 49%) failed to improve, and about 5% were worse in both groups (nine children developed new perforations). Differences between the azithromycin and amoxycillin groups in clinical failure rates were not significant.
No differences in clinical failure or failure to improve were indicated in a per-protocol analysis (children seen before Day 11 after commencement of treatment). Only four parents reported that their child had ear pain (three of the children were in the amoxycillin group). Fewer children in the azithromycin group than the amoxycillin group had runny nose (35% v 46%), but the difference was not significant, and about 4% of children in both groups had skin sores.
Clinical failure rates in children who had AOMwoP at baseline were 40% in the azithromycin group and 46% in the amoxycillin group. For children who had AOMwiP at baseline, clinical failure rates were 92% and 83%, respectively. (Clinical failure rates were 84% and 63%, respectively, at Day 12–21.)
There was no significant association between age group (< 2 years or ≥ 2 years) and clinical failure.
No significant difference in clinical failure was detected between azithromycin and amoxycillin groups for carriers or non-carriers of S. pneumoniae or NCHi at the end of therapy. Carriage of resistant S. pneumoniae or NCHi at baseline did not predict greater clinical failure. The risk difference (RD) in clinical failure between children carrying susceptible organisms (S. pneumoniae or NCHi) at baseline and those carrying resistant organisms at baseline was – 9%.
Complications and side effects. There were three serious adverse events that resulted in admission to hospital during the treatment period. The independent Data and Safety Monitoring Board found that these were not associated with the study interventions. No side effects (eg, allergic, gastrointestinal) led to cessation of treatment or withdrawal from the study.
New episodes of disease. In both groups, new perforations were detected in 3%–4% of children who had AOMwoP at baseline. Pain resolved in all children who had had pain at baseline, but four children developed “new” pain (Box 4).
Nasal carriage. Compared with children in the amoxycillin group, children in the azithromycin group had significantly lower carriage of S. pneumoniae and H. influenzae, lower carriage of S. pneumoniae with intermediate or full resistance to penicillin (RD, – 6% [but this difference was non-significant]), and significantly higher carriage of azithromycin-resistant S. pneumoniae (RD, 7%) (Box 5).
Ear discharge. The proportion of ear discharge cultures that were positive for S. pneumoniae and NCHi was about 25 percentage points lower in the azithromycin group (Box 5).
Compared with baseline, nasal carriage of S. pneumoniae was significantly lower in both groups at follow-up (azithromycin group RD, – 56% [95% CI, – 65% to – 47%]), and carriage of NCHi was also significantly reduced (azithromycin group RD, – 31% [95% CI, – 40% to – 21%]). In the azithromycin group, compared with baseline, carriage of S. pneumoniae with intermediate or full resistance to penicillin was lower at follow-up (10%) than at baseline (15%) (RD, – 5% [95% CI, – 12% to 2%]). In contrast, nasal carriage of azithromycin-resistant S. pneumoniae was higher at follow-up (10%) than at baseline (5%) (RD, 5% [95% CI, – 1% to 10%]).
In other published trials, an evidence summary and a meta-analysis that included azithromycin treatment of AOM, clinical response rates in groups receiving azithromycin have generally been 70% or higher.6-8,11,12,15-18 In a Cochrane review of short-course antibiotic treatment for AOM, outcomes of short courses of agents such as ceftriaxone or azithromycin were comparable with outcomes of longer courses of other antibiotics.8 Clinical trials assessing outcomes of single-dose azithromycin treatment11,12 have reported similarly high success rates. The Cochrane meta-analysis summary odds ratio for primary outcomes after 3–5 days’ treatment with azithromycin (n = 1347) compared with 10 days’ treatment with another antibiotic (n = 1246) was 1.09 (95% CI, 0.86 to 1.38). Success rates were about 86% in both azithromycin groups and comparison groups.8 In a single trial that reported outcome data for perforated or non-perforated eardrums of 28 evaluable patients with spontaneous perforation, there were more failures after 5 days of therapy than after 10 days of therapy.19
In our study, clinical success rates among Aboriginal children receiving azithromycin therapy for AOM were much lower than the rates reported in previous published studies. Reduced likelihood of cure is reported for patients with azithromycin-resistant S. pneumoniae (67%) compared with those with susceptible S. pneumoniae (90%).17 The rates of antimicrobial resistance found in our study (about 21%) do not explain the high proportion of clinical failures observed.
Poor adherence to recommended treatment regimens could explain poor clinical outcomes in the amoxycillin group. However, as the single dose of azithromycin was given under supervision, compliance with azithromycin treatment was close to 100%. In another of our studies in which compliance with twice-daily amoxycillin therapy was monitored, we estimated that 17/30 participants took less than half their recommended treatment.20 In that study, we found that AOM persisted in about three-quarters of participants and could not be explained by nasopharyngeal carriage of penicillin-resistant S. pneumoniae strains.20
The poor clinical outcomes associated with failure to eradicate NCHi in our study are consistent with clinical and bacteriological studies by Dagan et al showing clinical failure rates of 65%18 and 53%21 in patients with NCHi infections receiving azithromycin treatment.
We believe that persistent ear disease is related to high bacterial load in the nasopharynx.22 Antibiotics that eradicate otitis media pathogens and reduce bacterial load are needed for clinical success. While azithromycin was more effective at eradicating AOM pathogens than amoxycillin, nasal carriage of any S. pneumoniae or NCHi remained high at the end of therapy (65% in the azithromycin group and 92% in the amoxycillin group). For children with AOMwiP, ear discharge cultures were less likely to be positive after treatment with azithromycin. The observed association of better clinical outcomes with pathogen clearance and the greater impact of azithromycin on carriage (particularly for NCHi) led us to believe that clinical trials of longer courses of azithromycin treatment are required. The potential benefits for other important child health problems (eg, skin sores, runny nose and trachoma) should also be evaluated.
In the AATAAC study, most children with AOM at enrolment had an intact bulging tympanic membrane. However, as few had ear pain, it is likely that many children in this population will have undiagnosed AOM. This may explain the high rate of tympanic membrane perforation in this population. Further research is needed to determine the benefits of early detection of at-risk children as well as predictors of which children will progress to perforation.
3 Clinical outcomes at the end of therapy (between Day 6 and Day 11)
|
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
|
|||||||||||||
|
|
|
|
|
||||||||||||
|
|
|
||||||||||||||
|
|
|
|
|||||||||||||
|
Clinical failure according to nasal carriage of otitis media pathogens at the end of therapy |
|||||||||||||||
|
Nasal carriage of otitis media pathogens at baseline (both treatment groups combined) |
|
|
|||||||||||||
|
|
|||||||||||||||
Received 12 May 2009, accepted 17 August 2009
- Peter S Morris1,2,3
- Gaudencio Gadil1,2
- Gabrielle B McCallum1,2
- Cate A Wilson1,2
- Heidi C Smith-Vaughan1,2
- Paul Torzillo4
- Amanda J Leach1,2
- 1 Child Health Division, Menzies School of Health Research, Darwin, NT.
- 2 Institute of Advanced Studies, Charles Darwin University, Darwin, NT.
- 3 Northern Territory Clinical School, Flinders University, Darwin, NT.
- 4 Royal Prince Alfred Hospital, Sydney, NSW.
The National Health and Medical Research Council provided funding for our study but had no role in data analysis or manuscript preparation. We thank the families and children who participated in our study, the NT Department of Health and Families, the community health boards, and staff at the 16 clinics involved. We also thank the following AATAAC collaborators for their contributions to the field and laboratory data collection and analysis and assistance with reviewing our manuscript: Stephen Halpin and Joseph McDonnell (statistical analysts); Katrina Hodson and Susie Hopkins (clinical data collectors); Mandy Kennedy, Elizabeth Stubbs and Kim Hare (laboratory scientists); Jemima Beissbarth (laboratory scientist and data manager); Marius Puruntatameri (Indigenous Elder and study advocate); Kathy Currie (audiologist and study advocate); and Keith Edwards (paediatrician and study advocate).
None identified.
- 1. Morris PS, Leach AJ, Mellon G, Wilson C. Prevalence of otitis media (including AOM with perforation) in young Australian Aboriginal children from 29 remote communities: a 2001 pre-pneumococcal conjugate vaccine survey. Proceedings of the 8th International Symposium on Recent Advances in Otitis Media; 2003 Jun 3–7; Fort Lauderdale, Fla, USA.
- 2. Morris PS, Leach AJ, Halpin S, et al. An overview of acute otitis media in Australian Aboriginal children living in remote communities. Vaccine 2007; 25: 2389-2393.
- 3. Morris P, Ballinger D, Leach A, et al. Recommendations for clinical care guidelines on the management of otitis media in Aboriginal and Torres Strait Islander populations. Canberra: Office for Aboriginal and Torres Strait Islander Health, 2001.
- 4. Leach AJ, Morris PS, Mathews JD. Compared with placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatrics 2008; 8: 23.
- 5. Khurana CM. A multicenter, randomized, open label comparison of azithromycin and amoxicillin/clavulanate in acute otitis media among children attending day care or school. Pediatr Infect Dis J 1996; 15 (9 Suppl): S24-S29.
- 6. Schaad UB. Multicentre evaluation of azithromycin in comparison with co-amoxiclav for the treatment of acute otitis media in children. J Antimicrob Chemother 1993; 31 Suppl E: 81-88.
- 7. Principi N. Multicentre comparative study of the efficacy and safety of azithromycin compared with amoxicillin/clavulanic acid in the treatment of paediatric patients with otitis media. Eur J Clin Microbiol Infect Dis 1995; 14: 669-676.
- 8. Kozyrskyj AL, Hildes-Ripstein GE, Longstaffe SE, et al. Short course antibiotics for acute otitis media. Cochrane Database Syst Rev 2000; (2): CD001095.
- 9. Marcy M, Takata G, Chan LS, et al. Management of acute otitis media. Evid Rep Technol Assess (Summ) 2000; (15): 1-4.
- 10. De Diego JI, Prim MP, Alfonso C, et al. Comparison of amoxicillin and azithromycin in the prevention of recurrent acute otitis media. Int J Pediatr Otorhinolaryngol 2001; 58: 47-51.
- 11. Arguedas A, Loaiza C, Perez A, et al. A pilot study of single-dose azithromycin versus three-day azithromycin or single-dose ceftriaxone for uncomplicated acute otitis media in children. Curr Ther Res 2003; 64 Suppl 1: A16-A29.
- 12. Block SL, Arrieta A, Seibel M, et al. Single-dose (30 mg/kg) azithromycin compared with 10-day amoxicillin/clavulanate for the treatment of uncomplicated acute otitis media: a double-blind, placebo-controlled, randomized clinical trial. Curr Ther Res 2003; 64 Suppl 1: A30-A42.
- 13. Central Australian Rural Practitioners Association. CARPA standard treatment manual. 4th ed. Alice Springs: CARPA, 2003.
- 14. Stubbs E, Hare K, Wilson C, et al. Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media. Pediatr Infect Dis J 2005; 24: 423-428.
- 15. Dunne MW, Latiolais T, Lewis B, et al. Randomized, double-blind study of the clinical efficacy of 3 days of azithromycin compared with co-amoxiclav for the treatment of acute otitis media. J Antimicrob Chemother 2003; 52: 469-472.
- 16. Guven M, Bulut Y, Sezer T, et al. Bacterial etiology of acute otitis media and clinical efficacy of amoxicillin-clavulanate versus azithromycin. Int J Pediatr Otorhinolaryngol 2005; 70: 915-923.
- 17. Soley CA, Arguedas A. Single-dose azithromycin for the treatment of children with acute otitis media. Expert Rev Anti Infect Ther 2005; 3: 707-717.
- 18. Dagan R, Johnson CE, McLinn S, et al. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs azithromycin in acute otitis media. Pediatr Infect Dis J 2000; 19: 95-104.
- 19. Hendrickse WA, Kusmiesz H, Shelton S, Nelson JD. Five vs ten days of therapy for acute otitis media. Pediatr Infect Dis J 1988; 7: 14-23.
- 20. Gibney KB, Morris PS, Carapetis JR, et al. The clinical course of acute otitis media in high-risk Australian Aboriginal children: a longitudinal study. BMC Pediatr 2005; 5: 16-24.
- 21. Dagan R, Leibovitz E, Fliss DM, et al. Bacteriologic efficacies of oral azithromycin and oral cefaclor in treatment of acute otitis media in infants and young children. Antimicrob Agents Chemother 2000; 44: 43-50.
- 22. Smith-Vaughan H, Byun R, Nadkarni M, et al. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 2006; 6: 10-19.





Abstract
Objective: To compare the clinical effectiveness of single-dose azithromycin treatment with 7 days of amoxycillin treatment among Aboriginal children with acute otitis media (AOM) in rural and remote communities in the Northern Territory.
Design, setting and participants: Aboriginal children aged 6 months to 6 years living in 16 rural and remote communities were screened for AOM. Those diagnosed with AOM were randomly allocated to receive either azithromycin (30 mg/kg as a single dose) or amoxycillin (50mg/kg/day in two divided doses for a minimum of 7 days). We used a double-dummy method to ensure blinding. Our study was conducted from 24 March 2003 to 20 July 2005.
Main outcome measures: Failure to cure AOM by the end of therapy; nasal carriage of Streptococcus pneumoniae and non-capsular Haemophilus influenzae (NCHi).
Results: We followed 306 of 320 children (96%) allocated to the treatment groups. Single-dose azithromycin did not reduce (or increase) the risk of clinical failure (50% failure rate [82/165]) compared with amoxycillin (54% failure rate [83/155]) (risk difference [RD], – 4% [95% CI, – 15% to 7%]; P = 0.504). Compared with amoxycillin, azithromycin significantly reduced the proportion of children with nasal carriage of S. pneumoniae (27% v 63%; RD, – 36% [95% CI, – 47% to – 26%]; P < 0.001) and NCHi (55% v 85%; RD, – 30% [95% CI, – 40% to – 21%]; P < 0.001). Nasal carriage of S. pneumoniae with intermediate or full resistance to penicillin was lower (but not significantly so) in the azithromycin group (10% v 16%), but this group had significantly increased carriage of azithromycin-resistant S. pneumoniae (10% v 3%; RD, 7% [95% CI, 0.1% to 12%]; P = 0.001). Carriage of β-lactamase-producing NCHi was about 5% in both groups.
Conclusion: Although azithromycin reduced nasal carriage of S. pneumoniae and NCHi, clinical failure was high in both treatment groups. The possibility of weekly azithromycin treatment in children with persistent AOM should be evaluated.
Trial registration: Australian Clinical Trials Registry ACTRN 12609000691246.