Obstructive sleep apnoea syndrome is common and is associated with significant childhood morbidities
The consensus view is that OSAS affects about 3% of children, with 8%–12% snoring most nights.1,2 The peak prevalence occurs between the ages of 2 and 8 years, when lymphoid enlargement relative to upper-airway size is at its peak. However, upper-airway obstruction during sleep should not be attributed solely to large tonsils and adenoids. In fact, in many children they do not cause significant obstruction, and evidence to date suggests a poor correlation between tonsillar size and risk of OSAS.3 Other factors that may modulate the risk of obstruction include altered upper-airway tone, midface hypoplasia, allergic rhinitis, obesity and genetic factors.4 Therefore, finding large tonsils in a child who snores is not prima facie evidence of significant upper-airway obstruction during sleep.
Severe upper-airway obstruction in children is known to result in developmental delay, growth failure and cor pulmonale. What is less well appreciated are the more recently identified morbidities.5,6
Over the past 20 years, an extensive body of literature has detailed the effects of adult OSAS on daytime functioning. The areas affected include verbal and non-verbal intelligence, memory, attention, concentration, and executive functioning (ie, flexible analytical and problem-solving ability) and psychosocial functioning.7
The potential for similar effects in children was largely unstudied until a decade ago. There is now mounting evidence that disruption of children’s sleep architecture (ie, the normal pattern and sequence of stages of sleep) by repetitive episodes of hypoxia and arousal may result in similar deficits.8 The behavioural aspects most consistently reported include aggression, hyperactivity, inattention, anxiety and shyness, while learning, memory and executive functioning are the neurocognitive areas most affected.9 A direct relationship between the severity of OSAS in children and the resultant neurocognitive deficits has also been demonstrated.8 Areas of ongoing research in children include the effect of upper-airway obstruction on cardiovascular function (including systemic hypertension) and metabolic dysfunction.
The more controversial issue is that of snoring without significant changes in blood gas levels (primary snoring). This results from vibration of the soft tissues of the upper airway, and implies some degree of obstruction, albeit mild. Recent studies suggest that primary snoring may not be as innocuous as previously thought, with learning, neurocognitive and behavioural deficits being described in snoring children.6,10 For example, continuous snoring without intermittent hypoxia was found to be significantly associated with poor academic performance in mathematics (odds ratio [OR], 3.3), science (OR, 2.9), and spelling (OR, 4.5).10 The cause was postulated to be fragmentation of sleep architecture, although the authors did include in their analyses some children with what would be considered mild hypoxia.
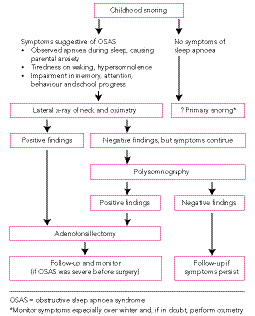
It is clear from the data of several studies that primary snoring cannot be confidently distinguished from OSAS on clinical history alone.11,12 While assessment is more straightforward in children with symptoms suggestive of severe obstruction, or in those with conditions that predispose to OSAS (ie, Pierre Robin syndrome, craniofacial syndromes or Down’s syndrome), most children with OSAS fall into the less severe category.
Features in the clinical history that can be helpful in deciding which children need surgery are listed in Box 1. Given that adenotonsillectomy carries a risk of morbidity, and even mortality, and that it costs about $40 million per year for adenotonsillectomies on 1% of Australian children, there is an imperative to target appropriate children for surgery.
On physical examination, adenoidal hypertrophy is suggested by a hyponasal voice and evidence of daytime upper-airway obstruction (eg, mouth breathing), while a muffled voice may result from tonsillar enlargement. Failure to thrive may occur in younger children, especially those with more severe obstruction, and growth parameters should be plotted.
In addition to the length of the soft palate, and the size of the tongue and oropharyngeal space, the tonsils and facial structure should always be assessed. Nasal patency should be checked, midface hypoplasia or micrognathia excluded, and a loud second heart sound (suggestive of increased right-heart pressure) ruled out. The presence of systemic hypertension and alterations in chest-wall shape should also be evaluated, the latter suggesting increased respiratory effort.
This provides useful information about the size of the adenoids and the postnasal space. Even if the postnasal space is patent while awake, its patency may diminish significantly during sleep, especially during rapid-eye-movement sleep when muscle tone is at its nadir.
This is a useful screening test because it is relatively simple to perform, is widely available, and has a positive predictive value of greater than 90%.13 However, its limitations as a diagnostic tool need to be highlighted. In a recent study of a cohort of 349 children referred for assessment of snoring to a tertiary sleep laboratory, screening oximetry had a negative predictive value of only 47% when compared with polysomnography.13 Therefore, negative findings can not be used to confidently exclude OSAS. Another important factor is that different types of oximeters may have varying performance characteristics, especially with regard to averaging times and movement artefact. Thus, the presence and severity of desaturation episodes may vary according to the specific type of equipment used in the study. Furthermore, oximeters with long averaging times, or algorithms designed to automatically remove movement artefact, are more likely to record false-negative results.
Polysomnography is currently the gold standard for the detection and assessment of the severity of OSAS in children.14,15 Polysomnography is a simultaneous recording of multiple physiological parameters related to sleep and wakefulness (brain activity, eye movements, muscle activity, heartbeat, blood oxygen levels and respiration). Although polysomnography is often labelled as an expensive procedure, it is not significantly more expensive than other paediatric investigations, such as echocardiography. However, a restricting factor may be the limited number of paediatric sleep facilities in Australia.
As new research reveals significant morbidity, such as learning deficits, potentially associated with primary snoring,6 the critical cut-off level for treatment of childhood OSAS is regularly being reviewed. Many clinicians screen children with overnight oximetry if they have a history suggestive of OSAS. Children with abnormal oximetry findings16 are referred for adenotonsillectomy. If the oximetry findings are normal and the clinical history is suggestive of OSAS, a polysomnogram will delineate the degree of upper-airway obstruction. Polysomnography is especially helpful for evaluating the risk of perioperative complications of adenotonsillectomy in high-risk children (see section on Adenotonsillectomy).
In children undergoing polysomnography, the current consensus is that those with an obstructive apnoea–hypopnoea index of more than five events per hour should be referred for adenotonsillectomy, while surgical treatment is generally considered optional for those with an apnoea–hypopnoea index of three to five per hour. The latter group require ongoing clinical monitoring, with the recommendation for or against surgery more likely to be modified by the clinical history and physical findings.
Important issues that will improve the assessment of children for surgery include standardising the analysis and interpretation of paediatric sleep studies,14 and including factors other than just the obstructive apnoea–hypopnoea index, as it can be difficult to capture the severity of OSAS if interpretation is confined to a simple score. Children have a predisposition to long periods of obstructive hypoventilation rather than discrete apnoeas. This means that cut-off levels for apnoea–hypopnoea indices that reflect disease significance are generally much lower than for equivalent studies in adults.
Adenotonsillectomy is the most appropriate therapy for most children with OSAS, and may significantly reduce their behavioural, learning and neurocognitive deficits.17,18 In a cohort of 297 first graders who were performing poorly academically, Gozal found a 6- to 9-fold increase in the prevalence of OSAS. Adenotonsillectomy in the children who had OSAS resulted in a significant improvement in their academic performance in the following year,5 but there was no such improvement in those with OSAS whose parents declined treatment.
Lipton and Gozal, in a recent extensive review of the outcomes of adenotonsillectomy,19 reported the cumulative “cure” rate to be about 80%. (“Cure” is disappearance of symptoms and normalisation of overnight respiratory parameters.) They acknowledged that studies published to date are generally from tertiary referral centres and therefore more likely to include children with coexisting morbidities or underlying abnormalities. Outcomes in non-tertiary settings have not been well studied.20
The same review found morbidity rates of 18%–34% in children with OSAS having adenotonsillectomy,19 suggesting that the cost–benefit ratio for individual children needs to be carefully assessed. Complications that can occur in the postoperative period include upper-airway obstruction secondary to oedema, immediate or delayed haemorrhage, and pulmonary oedema. The risk of postoperative complications is highest in young children (ie, younger than 3 years) and those with severe OSAS (especially when combined with failure to thrive, right ventricular hypertrophy, and a history of prematurity), and in those with craniofacial abnormalities, especially midface hypoplasia.18 It is important that surgery in children who are at increased risk of postoperative complications is undertaken in units with specialised intensive care facilities.
Despite this, as the tonsils and adenoids represent a space-occupying lesion in the oropharynx, and the site of the adenotonsillar tissue is the commonest location of airway occlusion,21 adenotonsillectomy (rather than just the removal of the tonsils or adenoids) is the treatment of choice for childhood OSAS.
Data on the usefulness of nasal steroids in childhood upper-airway obstruction is limited. In a cohort of 25 children with moderate OSAS, nasal steroids (fluticasone) for a 6-week period reduced mean overnight oxygen desaturation episodes by over 50%; however, by the conclusion of the study, 46% of those treated with nasal steroids required adenotonsillectomy.22 The role of this therapy is yet to be defined, but some authorities argue that a trial of nasal steroid administration may be worthwhile in children with less severe OSAS. The optimal duration of treatment is not known.
Positive pressure support is usually reserved for children with persistent OSAS despite adenotonsillectomy, or for those with specific contraindications to surgery. Unlike adenotonsillectomy, it is not curative and may need to be used for many years. Treatment success depends on the expertise of an experienced “sleep team” that adjusts an appropriate mask to fit the child and optimise comfort, avoiding complications such as air leaks or pressure sores. The requisite pressure to correct upper-airway obstruction during sleep is titrated during polysomnography.
This method of treatment has been successful in children from infancy to teenage years, but depends in large measure on appropriate training of parents and children23 and regular follow-up. In children who have difficulty in breathing against the CPAP, bilevel ventilation is often successful. Bilevel ventilation allows positive pressure support on inspiration, which, unlike with CPAP, falls on expiration. Some concern exists regarding the potential for a CPAP mask, used from an early age, to cause midface hypoplasia, although the evidence is limited.24
Evidence-based practice tips
Primary snoring cannot be confidently distinguished from obstructive sleep apnoea syndrome on clinical history alone (III-2).11,12
The risk of upper airway obstruction does not depend on the size of the tonsils or adenoids per se, but on their relative size compared with that of the upper airway (III-2).3
Polysomnography is the gold standard method of detecting and assessing the severity of obstructive sleep apnoea syndrome in children (III-2).14,15
Adenotonsillectomy is the treatment of choice in children with proven obstructive sleep apnoea syndrome (III-2).17,18
There is a higher risk of perioperative problems in children who have severe obstructive sleep apnoea syndrome, comorbid medical conditions such as midface hypoplasia, or are younger than 3 years of age (III-2).19
Not all children with obstructive sleep apnoea syndrome are “cured” by adenotonsillectomy. About 20% will need ongoing evaluation and, possibly, further treatment (III-2).19
Levels of evidence (I–IV) are derived from the National Health and Medical Research Council’s system for assessing evidence.25
1 Symptoms of obstructive sleep apnoea syndrome suggesting the need for adenotonsillectomy
Snoring and:
Observed apnoea during sleep
Struggling to breathe during sleep. Parents report that they shake the child to prompt breathing during sleep
Parental anxiety, resulting in observing the child during sleep
Unusual sleeping positions (eg, the “sword swallower” position with neck extended), restless sleep
Slow to waken in the morning, tiredness on waking, hypersomnolence
Impairment in memory, attention and behaviour
- 1. Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance and behaviour in 4-5 year olds. Arch Dis Child 1993; 68: 360-366.
- 2. Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest 1995; 107: 963-966.
- 3. Wang RC, Elkins TP, Keech D, et al. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg 1998; 118: 69-73.
- 4. Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med 2001; 164: 16-30.
- 5. Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998; 102: 616-620.
- 6. Blunden S, Lushington K, Kennedy D, et al. Behaviour and neurocognitive performance in children aged 5-10 years who snore compared with controls. J Clin Exp Neuropsychol 2000; 22: 554-568.
- 7. Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep 2000; 23 Suppl 4: S102-S108.
- 8. Kennedy JD, Blunden S, Hirte C, et al. Reduced neurocognition in children who snore. Pediatr Pulmonol 2004; 37: 330-337.
- 9. Gozal D, O’Brien LM. Snoring and obstructive sleep apnoea in children: Why should we treat? Paediatr Respir Rev 2004; 5: S371-376.
- 10. Urschitz MS, Guenther A, Eggebrecht E, et al. Snoring, intermittent hypoxia and academic performance in primary school children. Am J Resp Crit Care Med 2003; 168: 464-468.
- 11. Carroll JL, McColley SA, Marcus CL, et al. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest 1995; 108: 610-618.
- 12. Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg 1995; 121: 525-530.
- 13. Brouillette RT, Morielli A, Leimanis A, et al. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000; 105: 405-412.
- 14. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996; 153: 866-878.
- 15. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002; 109: 704-712.
- 16. Nixon GM, Kermack AS, Davis GM, et al. Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry. Pediatrics 2004; 113 (1 Pt 1): e19-25.
- 17. Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr 1996; 155: 56-62.
- 18. Friedman BC, Hendeles-Amitai A, Kozminsky E, et al. Adenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndrome. Sleep 2003; 26: 999-1005.
- 19. Lipton AJ, Gozal D. Treatment of obstructive sleep apnea in children: do we really know how? Sleep Med Rev 2003; 7: 61-80.
- 20. Lim J, McKean M. Adenotonsillectomy for obstructive sleep apnoea in children. Cochrane Database Syst Rev 2003; (1): CD003136.
- 21. Arens R, McDonough JM, Corbin AM, et al. Upper airway size analysis by magnetic resonance imaging in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2003; 167: 65-70.
- 22. Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001; 138: 838-844.
- 23. Waters KA, Everett FM, Bruderer JW, Sullivan CE. Obstructive sleep apnea: the use of nasal CPAP in 80 children. Am J Respir Crit Care Med 1995; 152: 780-785.
- 24. Li KK, Riley RW, Guilleminault C. An unreported risk in the use of home nasal continuous positive airway pressure and home nasal ventilation in children: mid-face hypoplasia. Chest 2000; 117: 916-918.
- 25. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Handbook series on preparing clinical practice guidelines. Table 1.3: Designation of levels of evidence. Canberra: NHMRC, February 2000: 8. Available at: www.health.gov.au/nhmrc/publications/pdf/cp69.pdf (accessed Mar 2005).






Abstract
Always take a history of snoring and sleep disturbance when reviewing children in primary care, as there is evidence that episodes of hypoxia and arousal during sleep may result in deficits in memory, attention and behaviour, in addition to the well known sequelae of growth failure, developmental delay and cor pulmonale. Check for changes in behaviour affecting school progress.
To investigate for possible obstructive sleep apnoea syndrome (OSAS), clinical examination, lateral neck x-ray (adenoidal hypertrophy) and overnight oximetry (desaturation episodes) are useful screening tests, but oximetry is best used in conjunction with polysomnography. A negative oximetry test does not exclude OSAS.
Polysomnography is the best method for detecting and assessing the severity of OSAS in children, and is especially helpful for prioritising treatment and evaluating the risk of perioperative complications of adenotonsillectomy.
Adenotonsillectomy is thought to “cure” (ie, symptoms disappear and overnight respiratory parameters are corrected) in about 80% of children with OSAS. The remaining 20% need ongoing evaluation and treatment.
Further research is needed to determine the “true” prevalence of OSAS; what degrees of severity of upper-airway obstruction lead to morbidity requiring treatment; and whether the deficits in neurocognitive function associated with sleep-disordered breathing are fully correctable.