Management of atopic disease should extend beyond symptomatic treatment to identification and avoidance of allergen triggers and, if appropriate, immunotherapy.
Atopic disease occurs when the immune system is dysregulated, resulting in allergic inflammation. Genetic and environmental factors determine the dysregulation and the development of an atopic disease.
Atopic disease has a strong hereditary component — if both parents are affected by an atopic disease (or one parent and a sibling) 40% of offspring will be affected. The genetic influence is multifactorial — no single atopy gene has been identified. It is likely that children who have inherited the “atopy gene(s)” are more likely to become sensitised and to develop allergic inflammation when exposed to the specific environmental influences.1,2
Environment is an important determinant of atopic disease. Evidence for this includes the regional differences in disease incidence, the recent increase in prevalence of atopic disease, and that children born in a “low atopy” country tend to develop the atopic disease patterns of their host country when they migrate to a “high atopy” country.3
When do environmental factors exert their major effects? There may be a “window of opportunity” in the first few years of life when the developing immune system is particularly susceptible to being directed along an atopic pathway. However, given that adults who migrate can manifest atopic disease after a change in environment, immune changes may also occur at an older age.
Determining which environmental factors prevent or promote atopic disease is complex (Box 1). A credible suggestion for the environmental effects is the “hygiene hypothesis”, which postulates that reduced exposure to infectious diseases and microbial products (endotoxin), associated with a “cleaner” lifestyle in urbanised communities, has lessened the immune deviation of the developing immune system to a non-allergic response.4-6 Previously, allergen exposure was thought to be the most important environmental factor influencing this immune deviation. However, the role of allergen exposure in the development of atopic disease is yet to be clearly defined. For example, the “dose” of allergen exposure may be critical — early exposure to high levels of cat allergen may protect against the development of asthma, while low-level exposure may promote asthma.7 Further understanding of the factors that promote or prevent the development of atopic disease is needed, so that primary prevention interventions applicable to communities can be recommended.
The International Study of Asthma and Allergies in Childhood showed that there was marked variation in the global prevalence of atopic disease.3 This variation occurs not only between countries but also regionally within countries, with the highest prevalence being in westernised, industrialised countries. In these countries the prevalence of atopic disease has been rising since the latter part of the 20th century. Australian and New Zealand children have the fifth highest global rates of atopic disease (Box 2). Interestingly, recent Australian data in children suggest that the prevalence of asthma has plateaued, while that of eczema and rhinoconjunctivitis continues to increase.8
Children with atopic disease are frequently tested by means of a skin prick test or RAST to determine the presence of IgE antibodies specific to common environmental allergens. The indications for performing these tests are given in Box 3. The tests can answer the question “Does this patient have IgE antibodies to a particular allergen of interest?”. However, they do not necessarily answer the question “Is this allergen a cause of the patient’s symptoms?”. This is because positive results of skin prick tests and RAST frequently occur in asymptomatic subjects. To answer the second question, the results of the test have to be assessed together with the clinical history, and, in certain circumstances, in conjunction with either allergen reduction (eg, a trial of dust mite avoidance measures) or allergen challenge.9,10 However, a negative result of a skin prick test or RAST usually excludes the presence of an IgE-mediated allergy trigger (Box 4).
Skin prick testing (Box 5) can be done in children of any age, and it is a misconception that the test is less reliable or cannot be interpreted in toddlers and infants. Skin prick test results are given as the mean weal diameter (in mm) measured at 15 minutes. A skin prick test is positive if the weal diameter is 3 mm or greater in the presence of a negative control. When referring patients for skin prick testing, it is important to instruct them not to take antihistamine medication, as this will interfere with the test results. Steroids and β-agonists do not interfere with a skin prick test. Skin prick testing should only be performed by practitioners who have been trained in performing the test and interpreting the results.
RAST, which measures IgE antibody levels in serum, provides similar information to a skin prick test, but may be less sensitive or specific than a skin prick test (which is a measure of the actual reaction to the allergen including mediator release from mast cells), but it is more widely available and can be performed even if the child is taking antihistamines. The results are reported as a semiquantitative measure (low–moderate–high) or preferably as a level of specific IgE (IU/L).
In some children with atopy, skin prick testing and RAST are used to aid in the diagnosis of an IgE-mediated food allergy. There are published data for a restricted range of foods (egg, nuts, milk, soy, fish and wheat), correlating the level of specific IgE to the probability of a child reacting to that food;11,12 a particular form of RAST (CAP-RAST; Pharmacia, Michigan, USA), as well as skin prick testing, has been used (Box 6). If the weal diameter (usually > 6 mm) or RAST IgE level is above a predetermined cutoff, the child will have a greater than 95% probability of reacting to a formal food challenge. The level of specific IgE to a food allergen is best used together with the clinical history in the diagnosis of IgE-mediated food allergy. Specific IgE level can also be used as an indication of whether to perform a food challenge in children with a history of food allergy. Tests in children with non-IgE-mediated food allergy and food intolerances will not show the presence of specific IgE.
Currently, there are no known interventions that can cure allergic inflammation, and management is frequently symptomatic. However, it is important, if possible, to identify and avoid allergen triggers — in some circumstances this may allow for more effective symptomatic treatment. In specific circumstances, immunotherapy has a place for children older than 7 or 8 years of age with significant allergic rhinoconjunctivitis (see section on Immunotherapy, page 301).
Although atopic disease is commonly exacerbated by non-allergen triggers (eg, viral infection in asthma, skin irritants in eczema), identification and avoidance of potential allergens may significantly improve the disease.
Eczema is a major risk factor for food allergy.13 About 40% of infants with eczema will be sensitised to common food allergens, and a significant number of these will have a clinical food allergy. Food allergy should be suspected in infants with eczema who have:
severe eczema with a poor response to topical treatment;
recurrent vomiting — may be misdiagnosed as gastro-oesophageal reflux;
loose stools and failure to thrive;
a family history of food allergy; and
episodes of urticaria or anaphylaxis.
Exposure to allergens in food, or possibly breastmilk (Box 7), may exacerbate eczema.14 Food allergens (eg, ovalbumin, casein, nut) have been detected in breastmilk after maternal ingestion.15 Ingested allergens do not cause asthma or rhinoconjunctivitis, but acute wheeze or nasal symptoms may occur as part of a generalised allergic reaction.
Most food allergies in children are triggered by cow’s milk, egg, nut, sesame seed, soy, wheat and seafood proteins. These foods are basic components of children’s diets, so empirical avoidance is not recommended, as this may have adverse nutritional consequences, particularly in young children. Avoidance should only occur after a complete assessment to identify food allergies.
Once a food allergy has been identified, avoidance is the best treatment. However, avoiding food allergens can be difficult given their ubiquitous presence in foods, confusing food labelling, and hidden sources. Ideally, parents should consult a dietitian, who can educate them about avoiding food allergens and ensure that the child’s diet remains nutritionally adequate. This is particularly important for children with a cow’s milk allergy to maintain their calcium intake.
Children are constantly exposed to various aeroallergens, depending on geographic, climatic and local factors. The principal indoor allergens are house dust mite, animal dander and cockroach, while outdoor allergens include pollen (grass, tree, weed) and moulds. Measures to reduce exposure to indoor allergens are more effective than those to reduce exposure to outdoor allergens. Several measures may reduce house dust mite exposure (Box 8).16 Reducing animal dander exposure can be achieved by removing pets from households, but this seldom occurs. Outdoor allergens cannot be effectively avoided.
Measures to reduce exposure to house dust mites have not been shown to be effective in improving symptoms of asthma,17 but may be of benefit in rhinoconjunctivitis or eczema,18,19 although the results of studies have not been consistent.
Symptomatic treatment is indicated when there are unidentified allergen triggers, or allergen avoidance is impossible or results in only a partial response. The medications used are either anti-inflammatory and/or designed to block the effects of mediators released during the allergic or atopic response. The anti-inflammatory action of topical, inhaled, and sometimes oral, steroids is a mainstay of treatment. The recent development of leukotriene antagonists (montelukast) for use in mild persistent asthma, topical calcineurin inhibitors for eczema (tacrolimus and pimecrolimus), and monoclonal anti-IgE antibodies for asthma provide non-steroidal anti-inflammatory alternatives. In the future, other biological agents (such as monoclonal anti-IgE antibodies) will become available and offer an approach without associated side effects.
Unfortunately, there are a small number of children who, despite anti-inflammatory medication, have severe and disabling atopic disease. For children with severe eczema, oral immunosuppressive treatment may be indicated, and there are published case series and placebo-controlled studies on the use of cyclosporin and azathioprine.20,21 As mentioned previously, immunotherapy should be considered for older children with severe rhinoconjunctivitis.
The process of administering gradually increasing quantities of an allergen extract to induce tolerance was first used for grass-pollen-induced allergic rhinitis almost 100 years ago. Immunotherapy is only effective for IgE-mediated inhalant allergic disease and, although the exact mechanism is not known, immunotherapy is associated with the production of blocking antibodies, downregulation of lymphocytes and a decrease in allergen-specific IgE.
Case studies
A 19-month-old boy with food allergy
A mother brings her 19-month-old son to you after an allergic reaction. He has a history of eczema at 2 months of age. Subsequently, his mother avoided introducing egg and peanut products, as she had read they were bad for the eczema. She has given him wheat-containing foods with no exacerbation of the eczema. At 18 months of age, skin contact with raw egg white (hands and transferred to mouth) resulted in urticaria at the sites of contact that then became generalised and was associated with respiratory difficulty and wheeze. An ambulance was called and he was given inhaled bronchodilator, but he was not taken to hospital and adrenaline was not used.
Examination shows a healthy boy weighing 11 kg who has mild eczema. On skin prick testing, there is a positive reaction to egg white (10-mm weal), peanut (4-mm weal), and wheat (4-mm weal) but a negative reaction to cow’s milk.
Management
You explain to the mother that her son has an (IgE-mediated) allergy to egg, and that the generalised nature of his reaction to raw egg suggests he has ingested egg allergen. In view of the significant respiratory difficulty he experienced and his weight (> 10 kg), you provide the mother with an EpiPen Jr (150 μg) (CSL, Melbourne) and a written anaphylaxis action plan. You explain that an acute allergic reaction to food with respiratory difficulty is best treated with adrenaline.
The mother is sure that her son has never eaten peanut or peanut products, so you cannot be certain that he has peanut allergy. You explain that it is not uncommon for children with eczema and egg allergy to be sensitised to peanut without known ingestion, as exposure may have occurred via breast milk.
You reassure the mother that children often grow out of milk and egg allergy by 5 years of age (but this is less likely for peanut allergy). You arrange to reassess the boy yearly, with repeat skin prick testing to see whether he is growing out of any of the allergies. You suggest that he continue to eat wheat products, despite the positive skin prick test, as no clinical reaction to wheat has occurred. You advise continued avoidance of peanut products, and, if sensitisation persists, recommend that he have a peanut challenge test in hospital before school entry.
An 8-year-old boy with chronic rhinitis
A mother brings her 8-year-old son to you because of worsening snoring and difficulty breathing at night. He has a history of rhinorrhoea, nasal itch, and snoring from 4 years of age. She tells you that his symptoms have been progressing and that he now snores every night, affecting his sleep. She has become concerned about his difficult breathing and interrupted sleep. Although his symptoms occur all year round, they are worse in spring. He has been treated intermittently with promethazine and topical nasal steroids, with little effect. His teachers complain that he seems unable to concentrate at school. He does not have asthma, but has a history of eczema. The family has no pets and neither parent smokes. His mother has sought naturopathic advice, and he has undergone a Vega test,22 and now cow’s milk and wheat have been excluded from his diet.
Examination shows that he has a nasal crease, bilateral pallor, swelling of the inferior nasal turbinate bones and that he breathes through his mouth. On skin prick testing, he reacts to a rye grass pollen and house dust mite mix (10-mm weal). Skin prick testing is negative for common moulds and animal dander.
Management
You explain to the boy and his mother that he has symptoms of chronic rhinitis, which is likely to be allergic and triggered by exposure to seasonal pollen allergens and/or house dust mite. You recommend trying allergen avoidance measures for house dust mite (see Box 8) to see if he responds (indicating that he has a house dust mite allergy).
Seasonal allergens are harder to avoid and you recommend consistent symptomatic treatment with nasal corticosteroids, rather than oral antihistamines, for his symptoms of nasal obstruction. You suggest continuous treatment for at least 2 weeks.
If, despite treatment, snoring continues, you recommend an x-ray of his posterior nasal space to exclude adenoidal hypertrophy, and possibly investigation for obstructive sleep apnoea.
If, despite allergen avoidance and symptomatic treatment, intractable symptoms persist, you refer the boy to an allergist to institute and supervise immunotherapy, using rye grass and house dust mite extracts. As he does not have asthma, the risk of a serious adverse reaction is reduced.
There is evidence from numerous randomised controlled trials for the efficacy of injectable specific immunotherapy in rhinoconjunctivitis and/or asthma.23,24 The allergens evaluated include house dust mite, cat, and grass pollens, and there is limited evidence for some weed and tree pollens and moulds. As few trials have been conducted in children, injectable immunotherapy should only be done in school-age children with intractable rhinoconjunctivitis, and it is not recommended for treating asthma or in preschool children.
There is growing evidence that immunotherapy by other routes, particularly sublingual, provides some clinical benefits in selected subjects.25 However, the magnitude and duration of the effects, the benefit for asthma, and the effects on the underlying immunological mechanisms remain to be determined. Nevertheless, this route has obvious benefits for children and further research is awaited.
Established recommendations should be followed, and immunotherapy should be initiated and supervised by an allergist.26 Safety is a major concern. While injection-site reactions are common, systemic reactions are uncommon, but may result in bronchospasm and/or anaphylaxis, including hypotension, upper-airway oedema and collapse. Rarely, death may result, with most cases involving individuals with asthma, the use of highly purified and potent aqueous extracts and divergence from recommended protocols.
Many children with IgE and non-IgE-mediated food allergy have eczema. In addition, about a third of infants and young children with moderate to severe atopic eczema have been shown to have food-related triggers for their eczema. Several factors have been identified that suggest the occurrence of food allergy in infants and children with eczema (see list under the heading Ingested allergens on page 300).
Food allergy may be caused by the presence of specific IgE antibodies (in which case children have immediate reactions) or may be non-IgE (possibly T lymphocytes)-mediated reactions (which may exacerbate the eczema in a delayed fashion). The latter reactions can occur in the presence of a negative skin prick test or RAST to the exacerbating food.
Anaphylaxis is a severe and rapidly progressive generalised allergic reaction with multisystem involvement, including the respiratory and/or cardiovascular system. About 1 in 166 Australian school-aged children have experienced an episode of anaphylaxis, with the most common trigger being food, followed by insect venom, and, uncommonly, drugs.27 Atopic disease is not a risk factor for anaphylaxis triggered by drugs or insect venoms. However, food anaphylaxis is more common in children with eczema. Children with asthma are more likely to have severe episodes of anaphylaxis, regardless of the trigger. Death in childhood from anaphylaxis is a rare event, but is clustered in individuals with asthma.
The primary treatment of anaphylaxis is adrenaline, which can safely be administered intramuscularly in all children.28 Children with anaphylaxis should have an EpiPen, and should know how to avoid the trigger (if identified). They should be referred to an allergist for review. Australian guidelines for EpiPen prescribing have been published (www.allergy.org.au/anaphylaxis/epipen_guidelines.htm), as have anaphylaxis action plans (www.allergy.org.au/aer/infobulletins/posters/Anaphylaxis_plan_(child)_Au.pdf). An EpiPen should only be prescribed in the context of an anaphylaxis action and management plan, which must include education on its correct use. EpiPens have recently become available through the Pharmaceutical Benefits Scheme via authority prescription (see www.health.gov.au/pbs/general/listing/pbacrec/jun03/adrenali.htm).
Education of teachers and other school staff in anaphylaxis prevention and management is important and is included in the first-aid training programs conducted in many states.
As a result of the complexities surrounding environmental influences, including which potential parents should be targeted, it is not possible to make definitive recommendations on allergen avoidance to prevent atopic disease. Currently, the following factors play a role:
Breastfeeding: Exclusive breastfeeding for 4–6 months exerts some protective effect.29 This protection may persist for at least the first decade of life. However, the protective effects are relatively modest and have not been confirmed by all studies. Breastfeeding might reduce atopic disease by favouring the development of gut flora populations of bifidobacteria and lactobacilli, which appear to be protective.30
Solid food: Introduction of all solids should be delayed until after the age of 6 months, and the more highly allergenic foods — egg, peanuts, tree nuts, and fish — can be delayed for 2–3 years to lessen eczema and food allergy. It seems clear that the beneficial effects of delaying solids are restricted to the first few years of life, and that there is no significant benefit observed by the school-age years.
Fish oils: Observational studies show that one of the strongest protective factors for respiratory symptoms in children is inclusion of oily fish (which have a high content of omega-3 fatty acids) in the diet.31,32 A recent trial of dietary fish oil supplementation from birth showed a significant reduction in allergic cough, but not wheeze, at 3 years of age.
Smoking: Parents should refrain from smoking, as smoking increases the risk of recurrent wheeze and asthma.
Research is being conducted into ways of preventing asthma in atopic children with eczema. Interventions under investigation include pharmacological measures such as the regular use of a non-sedating antihistamine, allergen avoidance, dietary interventions and immunotherapy. Results are awaited with interest and, if effective, such interventions may have significant public health benefits.
It is fortunate that the recent increasing prevalence of atopic disease has coincided with an improved understanding of the basic pathogenic mechanism of these conditions. With time, this should allow more effective preventive and management strategies to be developed. Management of children with atopic disease should not be confined to symptomatic treatment, but should extend to identification and avoidance of allergen triggers and, if appropriate, immunotherapy. Prevention of atopic disease in high-risk infants and prevention of asthma in children with eczema are areas of current research.
Evidence-based practice tips
For women at high-risk of having an atopic child, recommending avoidance of food antigens during pregnancy is unlikely to reduce substantially the child's risk of atopic diseases, and such a diet may adversely affect maternal and/or fetal nutrition (I).14
Chemical and physical methods aimed at reducing exposure to house dust mite allergens do not improve asthma symptom scores, medication use or early morning peak flow measurements (I).17
There is no evidence to support feeding with a hydrolysed formula for the prevention of allergy in preference to exclusive breastfeeding (I).33
In high risk infants who are unable to be completely breast fed, there is evidence that prolonged feeding with a hydrolysed compared with a cow's milk formula reduces infant and childhood allergy and infant cow’s milk allergy (I).33
Feeding with a soy formula should not be recommended for the prevention of allergy or food intolerance in infants at high risk of food allergy or food intolerance (I).34
Levels of evidence (I–IV) are derived from the National Health and Medical Research Council’s system for assessing evidence.35
1 Perinatal and early childhood factors associated with preventing or promoting atopic disease
Factors preventing |
Factors promoting |
||||||||||||||
|
|
||||||||||||||
|
|
||||||||||||||
|
|
||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
* See text for explanation. |
|||||||||||||||
2 Prevalence of atopic disorders among Australian children
Prevalence of: |
6–7 year olds |
13–14 year olds |
|||||||||||||
Eczema ever (current eczema) |
23% (11%) |
16% (10%) |
|||||||||||||
Asthma ever (current wheeze) |
27% (25%) |
28% (29%) |
|||||||||||||
Hayfever ever (current rhinitis) |
18% (12%) |
43% (20%) |
|||||||||||||
Data obtained from the International Study of Asthma and Allergy in Childhood (questionnaire-based survey of 10 914 children in Melbourne, Sydney, Adelaide and Perth).1 |
|||||||||||||||
3 Indications for determining specific IgE antibodies (by skin prick test or RAST) in atopic disease
Eczema
When house dust mite allergy is suspected as a trigger
When IgE-mediated food allergy is suspected, either as a trigger or an associated condition
Asthma
When determining the atopic status is important to support the diagnosis of asthma
Rhinoconjunctivitis
When house dust mite allergy is suspected as a trigger for perennial rhinitis
When determining the atopic status is important to support the diagnosis of allergic rhinoconjunctivitis
When immunotherapy is being considered, specific IgE determination is used to identify the allergen
4 Uses of skin prick tests (SPT) and radioallergosorbent testing (RAST)
Things SPT/RAST can tell us
That a patient is sensitised to an allergen
The likelihood of reacting after a food challenge (restricted range of foods)
That a patient is not sensitised to an allergen and therefore an IgE-mediated reaction to that allergen is very unlikely
Things SPT/RAST cannot tell us
The severity of a reaction if a sensitised patient were exposed
Whether the patient’s symptoms are caused by the allergen
5 Skin prick testing
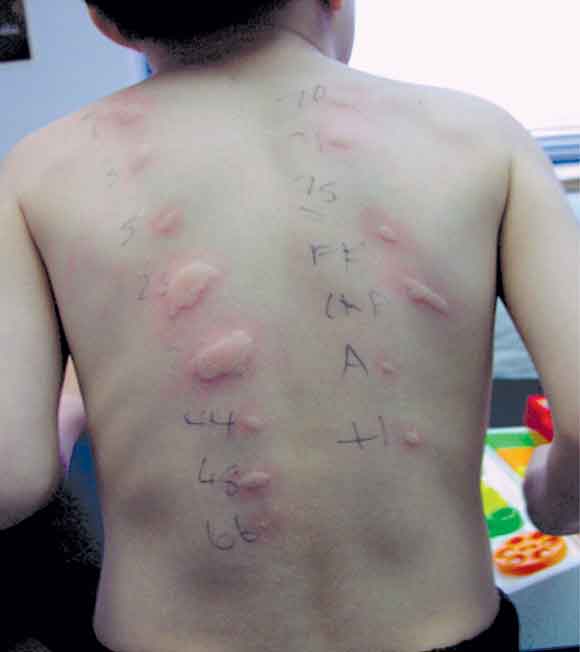
A skin prick test, showing multiple weal and flare reactions to common allergens in an atopic child with eczema.
6 Specific IgE level related to the probability of a food reaction

Food-specific IgE level (measured by ImmunoCAP-specific IgE blood test) and probability of reacting to that food after challenge.
7 Eczema in a 4-month-old infant


A: A 4-month-old boy with extensive eczema responded poorly to topical treatment, including corticosteroids. Exclusively breast fed, he had not yet commenced solids. Skin prick testing produced an 8-mm weal to egg protein and a 6-mm weal to cow’s milk protein, but was negative for peanut, wheat, soy and fish. Topical treatments were continued, and egg and cow’s milk were excluded from his mother’s diet under dietetic supervision. B: Child’s appearance 6 weeks later.
8 Measures to reduce house dust mite allergen exposure
Definitely useful
Encasing bedding in impermeable covers (dust mite covers) (this is the most important measure, as beds are a major source of house dust mite)
Washing bedding and clothes in hot water (> 56°C) every 1–2 weeks, which will destroy house dust mites and remove allergens
Removing or freezing stuffed toys
Reducing indoor relative humidity
Unlikely to be useful
Spraying carpets and mattresses with acaricides (dust mite sprays)
Washing of bedding in cold water with tea tree oil
- Michael S Gold1
- Andrew S Kemp2
- 1 Department of Paediatrics, Adelaide University, Women’s and Children’s Hospital, Adelaide, SA.
- 2 Department of Allergy, Immunology and Infectious Diseases, and Discipline of Paediatrics and Child Health, The Children’s Hospital Westmead and the University of Sydney, Sydney, NSW.
- 1. Nickel R, Lau S, Niggemann B, et al. Messages from the German Multicentre Allergy Study. Pediatr Allergy Immunol 2002; 13 Suppl 15: 7-10.
- 2. Halapi E, Hakonarson H. Recent development in genomic and proteomic research for asthma. Curr Opin Pulm Med 2004; 10: 22-30.
- 3. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998; 351: 1225-1232.
- 4. Check E. Link from hygiene to allergies gains support. Nature 2004; 428: 354.
- 5. Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002; 347: 869-877.
- 6. Ponsonby AL, Couper D, Dwyer T, et al. Relationship between early life respiratory illness, family size over time, and the development of asthma and hay fever: a seven year follow up study. Thorax 1999; 54: 664-669.
- 7. Almqvist C, Egmar AC, Hedlin G, et al. Direct and indirect exposure to pets — risk of sensitization and asthma at 4 years in a birth cohort. Clin Exp Allergy 2003; 33: 1190-1197.
- 8. Robertson CF, Roberts MF, Kappers JH. Asthma prevalence in Melbourne schoolchildren: have we reached the peak? Med J Aust 2004; 180: 273-276. <MJA full text>
- 9. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol 1999; 103: 981-989.
- 10. Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol 1984; 74: 26-33.
- 11. Hill DJ, Hosking CS, Reyes-Benito LV. Reducing the need for food allergen challenges in young children: a comparison of in vitro with in vivo tests. Clin Exp Allergy 2001; 31: 1031-1035.
- 12. Sampson HA. Use of food-challenge tests in children. Lancet 2001; 358: 1832-1833.
- 13. Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998; 101(3): E8.
- 14. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy and/or lactation for preventing or treating atopic disease in the child (Cochrane review). The Cochrane Library, Issue 3, 2004. Chichester, UK: John Wiley & Sons, Ltd.
- 15. Cavagni G, Paganelli R, Caffarelli C, et al. Passage of food antigens into circulation of breast-fed infants with atopic dermatitis. Ann Allergy 1988; 61: 361-365.
- 16. Vanlaar CH, Peat JK, Marks GB, et al. Domestic control of house dust mite allergen in children’s beds. J Allergy Clin Immunol 2000; 105(6 Pt 1): 1130-1133.
- 17. Gøtzsche PC, Johansen HK, Burr ML, Hammarquist C. House dust mite control measures for asthma (Cochrane review). The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
- 18. Sheikh A, Hurwitz B. House dust mite avoidance measures for perennial allergic rhinitis (Cochrane review). The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
- 19. Holm L, Bengtsson A, van Hage-Hamsten M, et al. Effectiveness of occlusive bedding in the treatment of atopic dermatitis—a placebo-controlled trial of 12 months’ duration. Allergy 2001; 56: 152-158.
- 20. Salek MS, Finlay AY, Luscombe DK, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol 1993; 129: 422-430.
- 21. Murphy LA, Atherton D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br J Dermatol 2002; 147: 308-315.
- 22. Katelaris CH, Weiner JM, Heddle RJ, et al, for the Australian College of Allergy. Vega testing in the diagnosis of allergic conditions [position statement]. Med J Aust 1991; 155: 113-114.
- 23. Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of asthma: a meta-analysis of prospective, randomized, double-blind, placebo-controlled studies. Clin Ther 2000; 22: 329-341.
- 24. Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of allergic rhinitis: an analysis of randomized, prospective, single- or double-blind, placebo-controlled studies. Clin Ther 2000; 22: 342-350.
- 25. Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2003; (2): CD002893.
- 26. Specific allergen immunotherapy for asthma. A position paper of the Thoracic Society of Australia and New Zealand and the Australasian Society of Clinical Immunology and Allergy. Med J Aust 1997; 167: 540-544.
- 27. Boros CA, Kay D, Gold MS. Parent reported allergy and anaphylaxis in 4173 South Australian children. J Paediatr Child Health 2000; 36: 36-40.
- 28. Lieberman P. Use of epinephrine in the treatment of anaphylaxis. Curr Opin Allergy Clin Immunol 2003; 3: 313-318.
- 29. van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003; 58: 833-843.
- 30. Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107: 129-134.
- 31. Hodge L, Salome CM, Peat JK, et al. Consumption of oily fish and childhood asthma risk. Med J Aust 1996; 164: 137-140. <MJA full text>
- 32. Dunstan JA, Dipa PG, Mori TA, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 2003; 112: 1178-1184.
- 33. Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants (Cochrane review). The Cochrane Library, Issue 1, 2005. Chichester, UK: John Wiley & Sons, Ltd.
- 34. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants (Cochrane review). The Cochrane Library Issue 3, 2004. Chichester, UK: John Wiley & Sons, Ltd.
- 35. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Handbook series on preparing clinical practice guidelines. Table 1.3: Designation of levels of evidence. Canberra: NHMRC, February 2000: 8. Available at: www.health.gov.au/nhmrc/publications/pdf/cp69.pdf (accessed Feb 2005).





Abstract
A child with atopy produces IgE antibodies after exposure to common environmental allergens. The atopic diseases (eczema, asthma and rhinoconjunctivitis) are clinical syndromes each defined by a group of symptoms and signs.
Not all children with atopy will have atopic disease or develop symptoms after exposure to an allergen. Both genetic and environmental factors determine the development of atopic disease.
The presence of specific IgE antibodies to environmental allergens is determined with skin prick or radioallergosorbent testing in children with atopy. Test results should be interpreted in the context of the clinical history and further investigations (eg, allergen avoidance or challenge).
Management of atopic disease is frequently symptomatic, but it is important to avoid identified allergen triggers. Immunotherapy may be considered in selected school-age children with severe rhinoconjunctivitis.
Preventing atopic disease in high-risk infants and hindering progression of disease in children with established disease are the areas of active research.