BreastScreen Australia has provided free mammographic screening for women aged 50–69 years since 1991, but there is at present no national policy on the age at which screening, or regular invitations for screening, should cease.
Breast cancer mortality rises steeply with age (from 21 per 100 000 among women aged 40–49 years to 117, 146 and 197 per 100 000 for women aged 75–79, 80–84 and 85+ years, respectively).1 However, as older women have a higher risk of death from other causes, the benefits of mammographic screening are increasingly offset by competing causes of death. In addition, older women may experience the immediate downsides of screening (discomfort, anxiety, side effects of tests and treatment), but not live long enough to experience the benefits.
A meta-analysis of trials recruiting women aged 40–74 years of age has shown that mammographic screening decreases breast cancer mortality (relative risk reduction, 23%; 95% CI, 13%–31%),2 but only one trial, the Swedish Two County Study, recruited women up to 74 years of age. That study found a (statistically non-significant) relative risk reduction of 21% (relative risk [RR], 0.79; 95% CI, 0.51–1.22) in women over 70 years at recruitment.3 A subsequent analysis of women in the same study aged 65–74 years at recruitment found a statistically significant relative risk reduction of 32% (RR, 0.68; 95% CI, 0.51–0.89).4 A more modest relative risk reduction in women aged 70–74 (0.79) compared with women aged 65–69 (0.58) was attributed in part to lower participation by older women in screening (77% v 86%, respectively). These data relate to women first recruited to screening around 70 years of age. As most Australian women aged over 70 who attend for screening have already been screened,5 they are unlikely to experience a comparable benefit.
Using a previously published approach to screening evaluation,6 we aimed to examine the balance of benefits and harms, and the resource implications, of continuing to screen women past 70 years of age. Because of the paucity of trial data, we reviewed decision-analytic models of mammographic screening in older women to assess the benefit of screening.
"Exploding" a search term retrieves all records with the exact term as well as those with more specific terms in the hierarchy of medical subject headings (MeSH).
The search strategy was [explode mammography] OR [explode breast neoplasms AND explode mass screening] AND [explode decision support techniques OR explode cost-benefit analysis] AND model.mp (MEDLINE looks for that word in the title, abstract or MeSH heading).
The search strategy was applied to MEDLINE 1966 to July 2000, and 44 articles were retrieved. All abstracts were reviewed and the relevant articles obtained in full. (This search was repeated in January 2002, and an additional 13 citations were retrieved; none met the inclusion criteria.)
We also contacted the authors of all models published since 1995 to locate any other published models, and reviewed the reference lists of all the articles describing models.
Included were articles reporting decision-analytic or cost-effectiveness models of screening mammography, which provided age-specific results for women 70 years and over (and for younger women for comparison) and presented the results as estimated gains in life expectancy per woman screened, either with or without quality-of-life adjustment.
The included articles were critically appraised according to criteria outlined by Richardson and Detsky,7 with modification to include the suggestion of Justice et al8 — that models should be assessed for their transportability between populations and that, if valid, they should accurately predict events in populations other than the one in which the model was developed.
The models varied widely in their methods, assumptions, results and presentation. To provide a common outcome, we calculated the relative life-expectancy benefit (and relative quality-adjusted life-expectancy benefit when available). This was done by establishing a base benefit predicted by the model for younger women (generally women aged 50–69 years). The base benefit — the average benefit per five or per 10 years (depending on data presentation) for women aged 50–69 years — was obtained from the article reporting the model or from previous publications with the same model or modelling approach. The relative life-expectancy benefit (and/or relative quality-adjusted life-expectancy benefit), estimated in 5- and/or 10-year age groups for older women, was then expressed as a percentage of the base benefit. For comparison, if data were available, we calculated the relative benefit for women aged 40–49 years (as a percentage of the same base benefit).
To quantify the harms of screening, we contacted all Australian State and Territory breast-screening coordination units and requested statistical reports. BreastScreen Queensland provided actual numbers of women screened (82 731 women aged 40–85+ years screened in 1998), recalled, tested and diagnosed with cancer. These data were converted to age-specific rates per 10 000 women screened. All data in the balance sheets are for asymptomatic women attending subsequent screening rounds. Data for women undergoing open biopsy include all women having open biopsy and may include some women having open biopsy as treatment.
Our estimates of the cost-effectiveness of extending mammographic screening to women aged 70–79 years were calculated from the costs per quality-adjusted life-year (QALY) provided by Boer et al using the MISCAN model (MIcrosimulation SCreening ANalysis).9,10 This model is the most comprehensive of the cost-effectiveness models available, and estimates, for a two-yearly screening program, health effects and social costs of the primary process of screening, changes in diagnostic procedures, primary therapies, follow-up treatment, metastatic disease, terminal illness and breast cancer mortality. The MISCAN model provides two variants: an optimistic model (which assumes no further increase in preclinical duration10 after the age of 65) and a pessimistic variant (which assumes a further increase in preclinical duration, with age extrapolated from the trend in younger age groups). The QALYs are discounted at an annual rate of 5%, and costs have been converted from euros to Australian dollars.
Five models met the inclusion criteria.11-15 A summary of the critical appraisal is given in Box 1. The models broadly fell into two groups: those that used an estimated relative risk reduction for screening in older women,11-13 and those that used changes in the distribution of cancer stages at detection achieved by screening to model the impact on disease progression and mortality.14,15 Thus, the models incorporated a wide range of assumptions about the effect of screening on mortality or stage distribution, baseline risk of mortality from breast cancer, stage-specific survival rates, test sensitivity and specificity, frequency of diagnosis of ductal carcinoma in situ (DCIS), attendance rates and discount rates for benefits and costs.
Only two of the models12,14 included adjustment for the impact on quality of life caused by screening. In one study12 the quality-of-life adjustment was limited to health states after diagnosis of breast cancer (ie, impact of diagnosis and initial treatment and impact of metastatic disease). In the other,14 there was also adjustment for a decrement in quality of life for women who were screened but did not have cancer (for anxiety and discomfort from the mammogram for screen-negative women; and anxiety, inconvenience and discomfort of biopsy for women with a false-positive result).
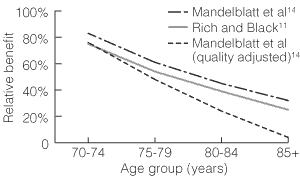
Relative life-expectancy benefits (and quality-adjusted relative life-expectancy benefits) of screening in women over 70 compared with women aged 50–69 years are shown in Box 2. For example, for the Rich and Black model,11 if we regard women in the base group (50–69 years) as experiencing 100% benefit from mammographic screening, then women 70–74 years will experience about three-quarters of that benefit, and women 75–79 years will experience about half the benefit (Box 2). When available, similar information is presented for quality-adjusted life expectancy. For example, from the model by Kerlikowske et al,12 we estimate that women aged 69–79 years will experience about 40% of the benefit experienced by women 50–69 years old, but, when quality of life adjustment is made, women 70–79 years experience only about 18% of the benefit experienced by younger women.
Thus, the life-expectancy benefit of screening mammography in older women appears to diminish with increasing age (Box 1). For women aged 70–79 years, the relative benefit ranges from an estimated 40% to 72% without quality-of-life adjustment, and from an estimated 18%–62% with quality-of-life adjustment. For women over 80 years, the relative benefit, estimated as 32%–39%, is further reduced when quality-of-life adjustment is made (14%). Relative effects of screening mammography for women in the 40–49-years age group were provided by only three studies,12,13,15 and only one12,16 included a quality-of-life-adjusted estimate.
The consequences of mammographic screening for older Australian women are summarised and compared with the consequences for younger Australian women in the balance sheet given in Box 4.
Of 10 000 Australian women undergoing a second or subsequent screening mammography, about 9600 women in each age group will be reassured that they do not have breast cancer and about 400 will be recalled for further tests. Of these, depending on age, about 70–112 women will undergo breast biopsy and 19–80 cancers will be detected. About 15%–20% of these cancers in each age group will be DCIS.
Box 5 shows the marginal cost effectiveness of extending the upper age limit for mammographic screening from 69 years to 79 years. Estimates range from $8119 (optimistic model) to $27 751 (pessimistic model) per QALY saved.
The benefit of screening women 70–79 years is about 40%–72% of that achieved in women 50–69 years, and declines further with increasing age and when quality-of-life adjustment is made. Extending screening to women 70–79 years is relatively cost-effective (similar to that of extending screening to women 40–49 years of age).
Age is the strongest risk factor for breast cancer, as indicated by the increasing number of cancers detected across age groups (Box 4). However, this increased risk, and therefore potential increased benefit, is mitigated by the reducing relative benefit from screening with age (Boxes 2, 3, 4).
Women who are at higher risk (family history or previous personal history of breast cancer) also have more to gain from screening. Conversely, women at low risk (eg, women who have low bone mineral density12 or a high risk of death from other causes) stand to benefit less from screening.14,17 Thus, those women least likely to participate in screening are, in fact, those least likely to benefit from screening. Therefore, whether women perceive themselves as in sufficiently good health to attend screening may be a good proxy for deciding whether screening is worthwhile.
Few quality-of-life-adjusted estimates were available. In the MISCAN model, which assesses cost-effectiveness,9,10 quality-of-life adjustment was done by including utilities (the weights used to adjust life-expectancy gains for impact on quality of life) for 16 health states arising from screening, which included screening attendance and attendance for follow-up of positive test results, as well as health states arising from the detection and treatment of breast cancer. However, we were unable to use this model in the assessment of benefit, as it does not provide outcomes in terms of life-expectancy gains per woman.
The two models providing information on average life expectancy with quality-of-life adjustment12,14 are consistent in showing that the benefit of screening is reduced when quality-of-life adjustment is made. Thus, women who are likely to be bothered by screening attendance, who will be made anxious by follow-up investigations and who will not easily tolerate a diagnosis of breast cancer and treatment are less likely to benefit from screening. Women who are diagnosed with DCIS are particularly likely to experience net harm if they are bothered by testing and treatment for cancer, as it is unlikely that DCIS would impact on their lifespan.18
In particular, the adverse effects of treatment need to be considered. Based on a large, representative 1990 sample, mortality rates for mastectomy are reported as 1.5% for women over 75 years (mean age, 81 years) and 0.5% for women under 75 (mean age, 57 years).19 Substantial postoperative morbidity has also been reported for between 7% and 20% of elderly women undergoing mastectomy.20,21 On the other hand, in terms of emotional and psychological effects, older women (over 50 years of age at diagnosis) may experience significantly less quality-of-life disruption (including emotional well-being, breast cancer concern, depression and disease-specific intrusive thought) than younger women.22 In short, more information about the impact of screening on quality of life in older women is urgently needed. Further, women's views about the balance of the benefits versus the adverse impacts of screening on quality of life are important when a decision to continue screening (or not) is made (Box 6).
There is clearly great uncertainty in the estimates of benefit from screening. The diversity of results of the models indicates the impact of differing assumptions and methods. Only with randomised trials can the likely effect size be quantified without bias. In recognition of the inherent uncertainty, we have presented range-of-benefit estimates. Further, there are known State-to-State variations in the delivery of screening services.5,23 Nevertheless, decisions need to be made and the estimates provided here give some indication of the likely effects of screening mammography in older women.
The conversion of costs per QALY estimates from Dutch data (given in euros) to Australian dollars provides a rough estimate of the relative efficiency (marginal cost-effectiveness) of extending biennial screening for women from ages 50–69 years to 50–79 years.9,10 Patterns of health service use and health costs are likely to be different between countries, but this is unlikely to have a substantial impact on cost-effectiveness ratios.
From an economic point of view, the central question is whether extending mammographic screening represents value for money. This is a value judgement based on the relative (marginal) cost of achieving health gain. One approach to answering this question is to compare the relative cost effectiveness of extending mammographic screening (from the current 50–69 years) to include women aged 40–49 years. In a previous study, we calculated (using the MISCAN model) the marginal cost of screening women from 40 years of age as between $24 000 and $65 000 per life-year gained.24 Although these cost-effectiveness ratios are not adjusted for "quality of life", the results show that extending screening to women aged 70–79 years is probably at least as cost-effective as extending screening to women aged 40–49 years.
1: Critical appraisal of decision-analytic models estimating life-expectancy gains from mammographic screening in older women (tinted entries indicate criterion is met)
Study |
Were important strategies included? |
Was the potential impact of uncertainty in the evidence determined? |
How strong is the evidence? |
Do the probabilities fit the Australian population? |
Do the utilities* reflect the values of older Australian women? |
||||||||||
Rich and Black 200011 |
Yes — compares screening from 50 years to end of life with no screening |
No sensitivity analyses done |
Assumes relative risk reduction is the same as in trials of women under 70 |
Probably, but incidence and mortality rates are higher in the US than in Australia. Model not tested in any other population |
No adjustment for quality of life |
||||||||||
Kerlikowske et al 199912 |
Yes — compares stopping screening at 69 years with stopping at 79 years |
Sensitivity analyses were done for varying discount rates,† relative risk reductions and breast cancer mortality rates |
Assumes range of relative risk reduction from 22% to 32% |
Probably, but incidence and mortality rates are higher in the US than in Australia. Model not tested in any other population |
Unknown — uses utilities only for two states: life after treatment and life with metastatic disease |
||||||||||
Kattlove et al 199513 |
Yes — compares biennial screening from 40 to 74 years with no screening |
No sensitivity analyses done |
Assumes relative risk reductions from Swedish Two County Study |
Not known |
No adjustment for quality of life |
||||||||||
Mandelblatt et al 199214 |
Yes — compares screening for women 65 years or more with no screening |
Sensitivity analyses for adverse impact on quality of life, breast cancer incidence, perioperative death, sensitivity and specificity of mammography and stage distribution of detected cancers |
Assumes US breast cancer stage distribution and stage-specific survival data |
Not tested in other populations |
Probably — utility data for impact of screening on women with negative and positive results of screening, but based on US women aged 50–74 years |
||||||||||
Eddy 198915 |
Yes — compares screening for women 40–75 years with no screening |
Only sensitivity analysis was inclusion of 5% discounting |
Assumes US stage and survival data plus trial data |
Model applied to UK and Malmö trial data and predicted greater than observed declines in mortality (model assumes annual screening and 100% compliance) |
No adjustment for quality of life |
||||||||||
* Weights used to adjust life-expectancy gains for impact on quality of life. |
|||||||||||||||
2: Summary of available relative life-expectancy benefits and quality-adjusted relative life-expectancy benefits of screening women 70 years and over
Relative benefit (RB)/quality-adjusted relative benefit (QARB) |
Average relative benefit per decade |
||||||||||||||
70–74 y |
75–79 y |
80–84 y |
≥ 85 y |
40–49 y |
70–79 y |
80–89 y |
|||||||||
Rich and Black11 |
RB |
75% |
54% |
39% |
25% |
65% |
32% |
||||||||
Kerlikowske et al12 |
RB |
40%† |
42% |
40% |
|||||||||||
QARB |
18%† |
37% |
18% |
||||||||||||
Kattlove et al13 |
RB |
33%‡ |
0 |
||||||||||||
Mandelblatt et al14 |
RB |
83% |
61% |
45% |
32% |
72% |
39% |
||||||||
QARB |
76% |
48% |
24% |
4% |
62% |
14% |
|||||||||
Eddy15 |
RB |
78%–90%§ |
67%–80% |
78%–90%§ |
|||||||||||
* Benefit for base group is regarded as 100%. |
|||||||||||||||
3: Impact of age on relative life-expectancy benefit according to different models
4: Balance sheet of the consequences of screening for women over 40 years (per subsequent screening round)
40–49 y |
50–69 y |
70–79 y |
≥ 80+ y |
||||||||||||
Number of screening mammograms |
10 000 |
10 000 |
10 000 |
10 000 |
|||||||||||
Number of women recalled for imaging tests |
450 |
417 |
407 |
401 |
|||||||||||
Number of women proceeding to biopsy* |
70 |
85 |
112 |
87 |
|||||||||||
Fine needle biopsy |
44 |
47 |
61 |
44 |
|||||||||||
Core biopsy |
30 |
41 |
50 |
36 |
|||||||||||
Open biopsy |
22 |
33 |
43 |
36 |
|||||||||||
Number of cancers detected |
19 |
37 |
65 |
80 |
|||||||||||
Invasive |
16 |
29 |
54 |
66 |
|||||||||||
Ductal carcinoma in situ |
3 |
8 |
11 |
15 |
|||||||||||
Relative life-expectancy benefit |
42%–80% |
100% |
40%–72% |
32%–39% |
|||||||||||
Relative quality-adjusted life-expectancy benefit |
37% |
100% |
18%–62% |
14% |
|||||||||||
* Number of women undergoing biopsy of any kind. The sum of individual biopsy procedures is greater because some women have more than one biopsy. |
|||||||||||||||
5: The marginal cost per quality-adjusted life-year (QALY) (discounted at 5%) of mammographic screening beyond 69 years
Marginal cost per QALY |
|||||||||||||||
Extending screening to: |
Optimistic model* |
Pessimistic model† |
|||||||||||||
73 years |
$6788 |
$18 374 |
|||||||||||||
75 years |
$7342 |
$20 754 |
|||||||||||||
77 years |
$7708 |
23 620 |
|||||||||||||
79 years |
$8119 |
$27 751 |
|||||||||||||
* Assumes no further increase in preclinical duration10 after the age of 65 years. |
|||||||||||||||
Received 8 February 2026, accepted 8 February 2026
- Alexandra L Barratt1
- Les M Irwig2
- Glenn P Salkeld3
- Paul P Glasziou4
- Nehmat Houssami5
- 1 Department of Public Health and Community Medicine, University of Sydney, Sydney, NSW.
- 2 Department of Social and Preventive Medicine, School of Population Health, University of Queensland Medical School, Herston, QLD.
- 3 Sydney Square Breast Clinic, Sydney, NSW.
This work was funded in part by the NHMRC National Breast Cancer Centre. We thank Rob Boer, Karla Kerlikowske, and Scott Rich for comments on drafts of this article, particularly with respect to their models of the effectiveness of screening mammography; Jennifer Muller and Stephen Heim, BreastScreen Queensland, for comments in developing this work and for assistance in developing the balance sheets of the benefits and harms of screening; State and Territory Co-ordination Units of BreastScreen Australia for their statistical reports and offers of assistance; and Mieke Van Doeland, Australian Institute of Health and Welfare, for assistance in obtaining data from BreastScreen Australia services and other sources.
- 1. Australian Institute of Health and Welfare. Breast cancer screening in Australia 1996-1997. Canberra: Australian Institute of Health and Welfare, 1998.
- 2. Kerlikowske K, Grady D, Rubin SM, et al. Efficacy of screening mammography. A meta-analysis. JAMA 1995; 273: 149-154.
- 3. Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age. Cancer 1995; 75: 2507-2517.
- 4. Chen HH, Tabar L, Fagerberg G, Duffy SW. Effect of breast cancer screening after age 65. J Med Screening 1995; 2: 10-14.
- 5. BreastScreen Queensland. 1998 Annual Statistical Report. Brisbane: Queensland Health, 2000.
- 6. Barratt A, Irwig L, Glasziou P, et al. Users' guides to the medical literature. XVII. How to use guidelines and recommendations about screening. JAMA 1999; 281: 2029-2034.
- 7. Richardson WS, Detsky AS. Users' guides to the medical literature. VII. How to use a clinical decision analysis. A. Are the results of the study valid? JAMA 1995; 273: 1292-1295.
- 8. Justice A, Covinsky K, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999; 130: 515-524.
- 9. Boer R, de Koning HJ, van Oortmarssen GH, van der Maas PJ. In search of the best upper age limit for breast cancer screening. Eur J Cancer 1995; 31A: 2040-2043.
- 10. Boer R. In search of the best upper age limit for breast cancer screening [doctoral thesis]. From evidence to decision support in cancer screening. Applications of MISCAN models. Chapter 8. Rotterdam: Erasmus University, 1999.
- 11. Rich JS, Black WC. When should we stop screening? Eff Clin Pract 2000; 3: 78-84.
- 12. Kerlikowske K, Salzmann P, Phillips KA, et al. Continuing screening mammography in women aged 70 to 79 years. Impact on life expectancy and cost-effectiveness. JAMA 1999; 282: 2156-2163.
- 13. Kattlove H, Liberati A, Keeler E, Brook RH. Benefits and costs of screening and treatment for early breast cancer. Development of a basic benefit package. JAMA 1995; 273: 142-148.
- 14. Mandelblatt JS, Wheat M, Monane M, et al. Breast cancer screening for elderly women with and without comorbid conditions. Ann Intern Med 1992; 116: 722-730.
- 15. Eddy D. Screening for breast cancer. Ann Intern Med 1989; 111: 389-399.
- 16. Salzmann P, Kerlikowske K, Phillips K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann Intern Med 1997; 127: 955-965.
- 17. Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 1994; 120: 104-110.
- 18. Ernster VL, Barclay J, Kerlikowske K, et al. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med 2000; 160: 953-958.
- 19. Busch E, Kemeny M, Fremgen A, et al. Patterns of breast cancer care in the elderly. Cancer 1996; 78: 101-111.
- 20. Hunt KE, Fry D, Bland KI. Breast carcinoma in the elderly patient: an assessment of operative risk, morbidity and mortality. Am J Surg 1980; 140: 339-342.
- 21. Morrow M. Breast disease in elderly women. Surg Clin North Am 1994; 74: 145-161.
- 22. Wenzel LB, Fairclough DL, Brady MJ, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer 1999; 86: 1768-1774.
- 23. BreastScreen Victoria. 1998 Annual Statistical Report. Melbourne: BreastScreen Victoria, 2000.
- 24. Irwig L, Glasziou P, Barratt A, Salkeld G. Review of the effectiveness of mammographic screening of women aged 40-49 years. Sydney: NHMRC National Breast Cancer Centre 1997. Available at: www.nbcc.org.au (see Information, Early Detection) (accessed January 2002, longer available).





Abstract
Objective: To assess the (i) benefits, (ii) harms and (iii) costs of continuing mammographic screening for women 70 years and over.
Data sources and synthesis: (i) We conducted a MEDLINE search (1966 – July 2000) for decision-analytic models estimating life-expectancy gains from screening in older women. The five studies meeting the inclusion criteria were critically appraised using standard criteria. We estimated relative benefit from each model's estimate of effectiveness of screening in older women relative to that in women aged 50–69 years using the same model. (ii) With data from BreastScreen Queensland, we constructed balance sheets of the consequences of screening for women in 10-year age groups (40–49 to 80–89 years), and (iii) we used a validated model to estimate the marginal cost-effectiveness of extending screening to women 70 years and over.
Results: For women aged 70–79 years, the relative benefit was estimated as 40%–72%, and 18%–62% with adjustment for the impact of screening on quality of life. For women over 80 years the relative benefit was about a third, and with quality-of-life adjustment only 14%, that in women aged 50–69 years. (ii) Of 10 000 Australian women participating in ongoing screening, about 400 are recalled for further testing, and, depending on age, about 70–112 undergo biopsy and about 19–80 cancers are detected. (iii) Cost-effectiveness estimates for extending the upper age limit for mammographic screening from 69 to 79 years range from $8119 to $27 751 per quality-adjusted life-year saved, which compares favourably with extending screening to women aged 40–49 years (estimated at between $24 000 and $65 000 per life-year saved).Conclusions: Women 70 years and over, in consultation with their healthcare providers, may want to decide for themselves whether to continue mammographic screening. Decision-support materials are needed for women in this age group.