The known: Breast cancer screening around the world has switched from film to digital mammography. The impacts of this change on health outcomes are unknown.
The new: In NSW, increased cancer detection with the new technology was predominantly of ductal carcinomas in situ; the detection of invasive cancer initially declined slightly. The interval cancer detection rate also increased, particularly the detection of invasive cancers. An initial increase in the rate of recalls for further assessment was largely attributable to the increased false positive result rate.
The implications: The transition from film to digital mammography may have increased the detection of indolent cancers.
Breast cancer screening programs moved from film to digital mammography during the first decade of the 21st century, primarily for workflow reasons,1,2 but improved cancer detection was also anticipated.3 After the change, it was found that digital mammography indeed detected more cancers than film mammography,4,5,6,7 particularly ductal carcinomas in situ (DCIS), with smaller increases in invasive cancer detection.8 However, most studies found no change in the reporting of interval cancers, suggesting that increased screening detection might predominantly be of slower growing (or even non‐progressive) cancers.8 At the same time, the transition to digital mammography was accompanied by a clear increase in recall rates, mostly related to false positive findings.8
The only analysis of the transition from film to digital mammography undertaken in Australia9 was not a population‐level study. BreastScreen Australia is a government‐funded screening program in which women aged 50−74 years are invited for screening every two years; women aged 40–49 years can also participate but are not formally invited. Two or more radiologists (or specifically trained breast physicians) independently read two‐view mammograms; the results are combined in a single recommendation about the need for further assessment to determine the presence of breast cancer.10
Overseas comparisons of digital and film mammography have used cancer rates without adjustment for time. However, as the two screening modalities were used during different time periods, rate differences could be confounded by time‐dependent factors, such as the changing background risk of breast cancer.11 In Australia, the transition from film to digital mammography in BreastScreen was largely undertaken during 2009 and 2010. As sufficient time has since elapsed, we assessed the impact of the transition of the Australian national screening program to digital mammography in an interrupted time series analysis adjusted for confounding by temporal trends.12
Methods
The national breast screening program in Australia, BreastScreen Australia, commenced in 1988, and screening was nationally available by 1991.10 BreastScreen is nationally governed but, like most health care in Australia, it is implemented at the state level. A screening episode commences with the initial attendance for screening and includes any recalls for technical repeat screening or the assessment of abnormalities detected by screening mammography. A screening episode is completed when a recommendation is made to return the woman to routine screening or a cancer diagnosis is made. An invasive breast cancer or DCIS diagnosed after additional investigations is classified as a screen‐detected cancer. If cancer is not detected, it may be recommended that the woman return to two‐yearly, annual, or early screening (within six months). Cancers diagnosed after a recommendation to return to screening but before the end of the recommended screening interval are classified as interval cancers.8
Study cohort and data sources
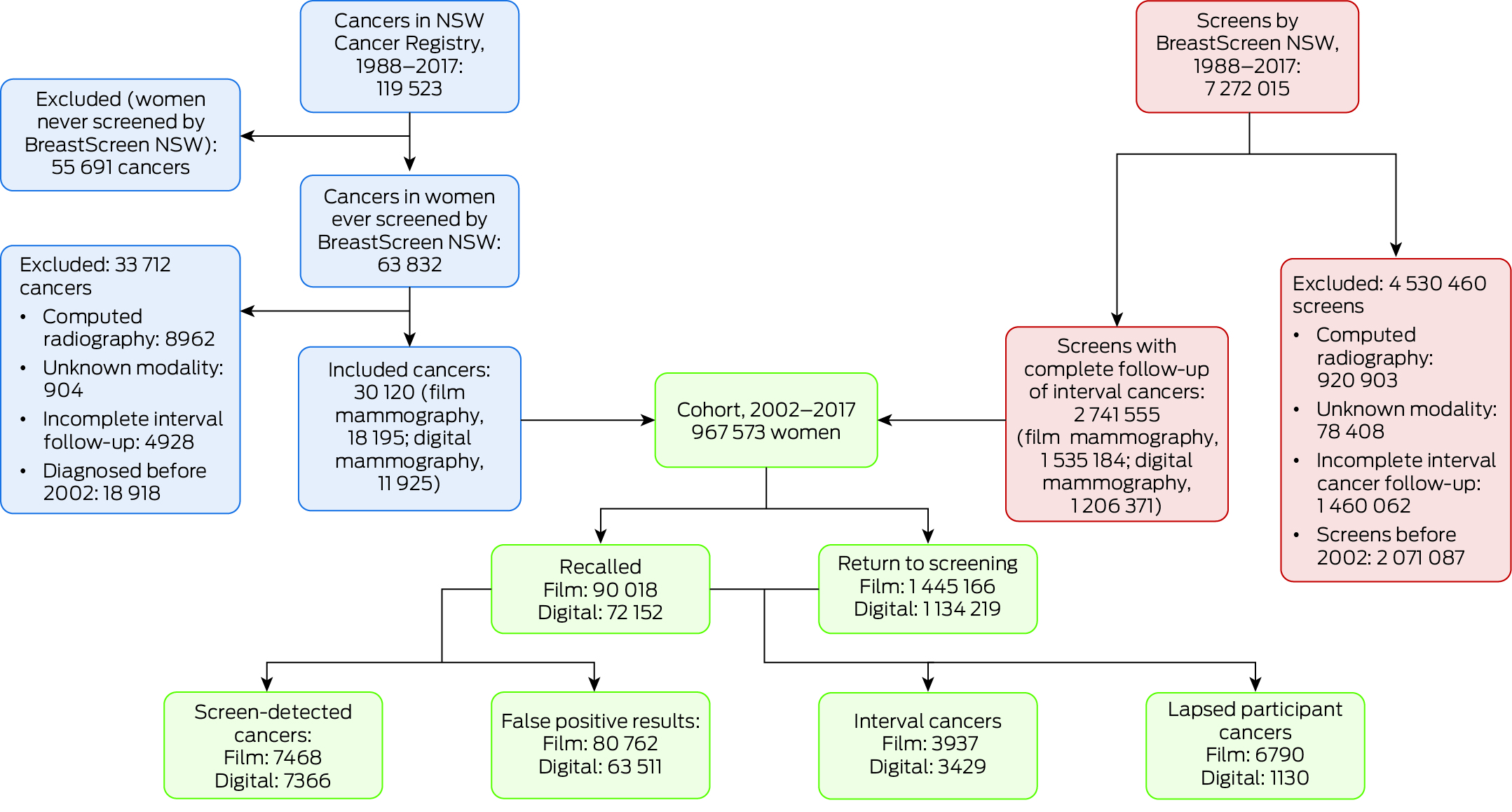
The study cohort has been described in detail elsewhere.13 Briefly, we used data linkage to construct a study cohort comprising two overlapping groups of women: all women screened by BreastScreen NSW from its inception (1 March 1988) to 31 December 2017; and all women aged 40 years or older (ie, screening‐eligible) diagnosed with DCIS or invasive breast cancer notified to the NSW Central Cancer Registry during 1 January 1988 – 31 December 2017. In 2024, New South Wales included an estimated 8.2 million people, or about one‐third of the Australian population.14
The BreastScreen NSW dataset comprises data for all program‐related mammography undertaken in NSW, including screening results, results of follow‐up investigations, and the final recommendations for each screening round. In order to achieve similar study time frames for film and digital mammography screening, we limited our study to women screened during 1 January 2002 – 31 December 2016; however, we used all BreastScreen NSW screening data since its inception to determine whether screening episodes for individual women during the study period were initial or subsequent screens.
BreastScreen NSW and NSW Central Cancer Registry data were probabilistically linked by the NSW Centre for Health Record Linkage (CHeReL).15 The registry uses pathology laboratory, hospital, radiotherapy and medical oncology departments, aged care facility, and Registry of Births, Deaths, and Marriages data to histologically verify all cases of breast cancer diagnosed in NSW residents, including DCIS. The linkage of the two datasets allowed us to determine the screening modality and subsequent outcomes for each woman with a high level of capture and accuracy.
Computed radiography screening was used only briefly by BreastScreen; it exposed women to higher radiation doses and provided lower quality images than digital mammography.16 We therefore excluded women screened using this modality from our analysis.
Outcomes
Screen‐detected breast cancers were defined as those diagnosed on the basis of a positive screening result; we report the number of women diagnosed with screen‐detected breast cancer, including DCIS, per 1000 screens. Interval cancers were defined as cancers diagnosed after a negative screening mammography result and before the next scheduled screening, or cancers that were symptomatic on a subsequent screen; we report the number of interval cancers, including DCIS, per 1000 screened women. The recall rate was defined as the number of women with positive screening results recalled for further assessment per 1000 screened women, and the false positive rate as the difference between the recall and screen‐detected cancer rates.
Statistical analysis
We initially included all screens undertaken by BreastScreen NSW during 2002–2016, regardless of whether the women had been invited to participate in screening. We stratified screens as initial or subsequent screening round screens, as the cancer detection rate differs by screening round.17 We stratified both screen‐detected and interval cancers as DCIS or invasive cancers, as the prognosis differs by cancer type.18,19 We excluded screens for which the follow‐up time was not at least as long as the interval to the next recommended screen; that is, insufficient for ascertaining whether an interval cancer had been detected. Cancers diagnosed beyond the time point for the next recommended screening were deemed to be “cancers in lapsed screening participants”, in line with BreastScreen terminology. We report summary statistics that include cancers in lapsed screening participants, but did not include these cases in our regression analyses. We report overall rates of screen‐detected cancers, interval cancers, recalls, and false positive findings per 1000 screens for film and digital mammography, each with 95% confidence intervals (CIs). We calculated unadjusted rate differences in logistic regression models with robust standard errors.
We assessed rate changes in interrupted time series analyses, using 2009 as the transition time point, the main period of transition from film to digital mammography. An interrupted time series is a strong quasi‐experimental design for estimating changes attributable to an intervention (here: change in screening modality). We compared outcome rates after the introduction of digital imaging with those predicted by temporal trends preceding its introduction, which reflect changes unrelated to the screening modality, such as screening women at a younger age in more recent years.12,20,21,22,23 We used segmented autoregression to statistically estimate aggregate changes in annual rates between the film and digital mammography periods. The models estimate the baseline temporal trend during the film period, the immediate effects of the switch from film to digital mammography, and the temporal trend after the introduction of digital mammography. We used the segmented regression equation:
Y = β0 + β1 * time (pre-intervention) + β2 + β3 * time (post-intervention)
β0 is the estimated baseline rate with film mammography; β1 is the estimated annual temporal change in rate during the film mammography period (2002–2009); β2 is the estimated change in rate attributed to the change to digital mammography; and β3 is the estimated additional change in rate during the digital mammography period (2009–2015). We report β0, β1, β2, and β3, overall and by screen type (initial or subsequent screens); we depict rates by time derived from independent models for initial and subsequent screens as graphs.20,24 We performed the linked data analyses in SAS 9.4, the interrupted time series analysis in SAS Studio 3.1 (PROC AUTOREG).
Ethics approval
The NSW Population and Health Service Research Ethics Committee approved the study (HREC 2019/ETH08688). Access to the datasets used was approved by their respective data custodians.
Results
During 2002–2017, BreastScreen NSW undertook a total of 5 200 928 screens; we excluded 920 903 computed radiography screens, 78 408 screens for which the modality was unknown, and 1 460 062 otherwise eligible screens for which interval cancer follow‐up was incomplete. Our study cohort comprised 967 573 women aged 40 years or older with breast cancers diagnosed during 2002–2017 who had been screened by BreastScreen NSW and for whom complete follow‐up information until the end of the recommended re‐screening interval was available (Box 1). Of the 2 741 555 screens included in our analysis, 1 535 184 used film mammography (2002–2010) and 1 206 371 used digital mammography (2006–2016) (Box 2). A total of 30 120 breast cancers were diagnosed in these women: 14 834 screen‐detected cancers and 7366 interval cancers, as well as 7920 lapsed screen participant cancers (Supporting Information, table 1).
Screen‐detected cancers
The screen‐detected cancer rate was 4.86 (95% CI, 4.75–4.97) cases per 1000 screens with film mammography (2002–2010) and 6.11 (95% CI, 5.97–6.24) cases per 1000 screens with digital mammography (2006–2016), an unadjusted difference of 1.24 (95% CI, 1.06–1.41) cases per 1000 screens. The invasive cancer rate increased by 0.70 (95% CI, 0.55–0.86) cases per 1000 screens, the DCIS rate by 0.53 (95% CI, 0.45–0.60) per 1000 screens (Box 3). In the time series model adjusted for temporal trends, the initial background rate was 4.65 per 1000 screens; it rose by 0.05 cases per 1000 screens per year during 2002–2009 (Box 4).
With the transition to digital mammography, the screen‐detected cancer rate increased by 0.07 cases per 1000 screens, the sum of the decline in the invasive cancer rate (–0.21 per 1000 screens) and the rise in the DCIS detection rate (0.28 per 1000 screens). During 2009–2015, the screen‐detected cancer rate increased by 0.18 cases per 1000 screens per year, comprising rises of 0.16 invasive cancers and 0.01 DCIS per 1000 screens per year (Box 4).
For initial screens, the screen‐detected cancer rate increased by 0.28 cases per 1000 screens followed by an increase of 0.06 cases per 1000 screens per year. For subsequent screens, the screen‐detected cancer rate increased by 0.04 cases per 1000 screens followed by an increase of 0.19 cases per 1000 screens per year (Box 4, Box 5).
Interval cancers
The interval cancer rate was 2.56 (95% CI, 2.48–2.64) cases per 1000 screens with film mammography (2002–2010) and 2.84 (95% CI, 2.75–2.94) cases per 1000 screens with digital mammography (2006–2016), an unadjusted difference of 0.27 (95% CI, 0.15–0.40) cases per 1000 screens. The invasive cancer rate increased by 0.18 (95% CI, 0.06–0.29) cases per 1000 screens, the DCIS rate by 0.09 per 1000 screens (95% CI, 0.05–0.13) (Box 3). In the time series model adjusted for temporal trends, the initial background rate was 2.52 cases per 1000 screens; it rose by 0.01 cases per 1000 screens per year during 2002–2009 (Box 4).
With the transition to digital mammography, the interval cancer rate increased by 0.75 cases per 1000 screens, comprising rises in the invasive cancer (0.69 per 1000 screens) and DCIS detection rates (0.08 per 1000 screens). During 2009–2015, the interval cancer detection rate declined by 0.13 cases per 1000 screens per year, entirely attributable to the decline in the interval invasive cancer detection rate (Box 4).
Recalls and false positive results
The recall rate was 58.6 (95% CI, 58.3–59.0) per 1000 screens with film mammography and 59.8 (95% CI, 59.4–60.2) per 1000 screens with digital mammography, an unadjusted difference of 1.17 (95% CI, 0.60–1.73) cases per 1000 screens. The recall rate for initial screens increased from 102 (95% CI, 101–104) to 130 (95% CI, 128–131) per 1000 screens, an unadjusted difference of 27.4 (95% CI, 25.4–29.4) recalls per 1000 screens. The recall rate for subsequent screens declined from 51.4 (95% CI, 51.0–51.8) to 48.6 (95% CI, 48.1–49.0) per 1000 screens, an unadjusted difference of –2.83 (95% CI, –3.39 to –2.27) recalls per 1000 screens (Box 3).
Most recalls were related to false positive findings, the overall rate of which did not change (both modalities: 52.6 per 1000 screens; unadjusted difference between digital and film mammography, 0.03 [95% CI, –0.49 to 0.57] false positive findings per 1000 screens). The false positive finding rate for initial screens increased from 94.6 (95% CI, 93.3–95.8) to 120 (95% CI, 118–122) per 1000 screens (unadjusted difference, 25.5 [95% CI, 23.5–27.5] per 1000 screens); the rate for subsequent screens declined from 45.6 (95% CI, 45.3–46.0) to 41.8 (95% CI, 41.4–42.2) per 1000 screens (unadjusted difference, –3.85 [95% CI, –4.37 to –3.32] per 1000 screens (Box 3).
In the interrupted time series adjusted for temporal trends, the initial background recall rate was 55.6 per 1000 screens; it rose by 0.78 cases per 1000 screens per year during 2002–2009. With the transition to digital mammography, the recall rate increased by 8.02 per 1000 screens during 2009–2015, it declined by 2.97 cases per 1000 screens per year (initial screens, –14.2 cases per 1000 screens per year; subsequent screens, –6.34 cases per 1000 screens per year) (Box 4, Box 7).
The temporal changes in false positives were similar to those seen in recall rates with an immediate increase of 7.16 per 1,000 screens, and a yearly decrease of 2.95 per 1000 screens with digital mammography. However, the pattern was more pronounced in subsequent screens than initial screens. (Box 4, Box 7).
Age and screening round
For all screens, the difference between film and digital mammography screen‐detected cancer rates declined by ten‐year age group from 40–49 to 60–69 years, but rose again for women aged 70 years or older. The difference for interval cancer detection was smaller for women aged 50–69 years than for those aged 40–49 years, but was highest for women aged 70 years or older. Recall rates declined with increasing age (Supporting Information, table 2).
Discussion
The BreastScreen NSW transition from film to digital mammography during 2006–2010 was accompanied by an increase in the screen‐detected cancer rate, especially during initial screens; the increase was primarily attributable to the higher DCIS detection rate. Our interrupted time series analysis, adjusted for secular changes in cancer diagnosis rates, indicated that the screen‐detected invasive cancer rate declined at the transition point before rising again. If most of the additional screen‐detected cancers were clinically important, interval cancer rates would be expected to decline, but they initially increased, particularly for subsequent screens. Some additionally detected cancers might otherwise have been detected at subsequent screenings, or they might have been detected later as interval cancers, especially invasive cancers. It is also possible that some cancers would not ever have been detected during life, particularly low grade DCIS, which may indicate overdiagnosis since the transition. The reason for the increase in the interval cancer detection rate at the transition is unclear, but the initial decline in the screen‐detected invasive cancer rate could be one factor.
Digital mammography provides better image quality and reduces the radiation exposure of screened women compared with film‐based screening, and also improved workflow efficiency.25,26 However, the high contrast and resolution of digital mammography not only accentuates lesions, but also normal tissue architecture. Although lesions may be easier to distinguish, reading and interpreting digital mammograms requires training.27 The increased recall rate immediately after the transition to digital mammography in NSW may indicate that radiologists saw lesions and tissue structures that had been less visible in film mammography. With continuous changes in screening technology, and the need for a period of learning after each change, recall rates may not stabilise if new technologies continue to be introduced.28 The increase in the recall rate was especially marked for women aged 40–49 years. Recall and false positive finding rates are generally higher in this age group than for older women, partly because of denser breast tissue in younger women.8 We found that the increases in the screen‐detected cancer rate was greatest for women aged 40–49 years or 70 years or older, while the increase in interval cancer detection rate was greatest for women aged 70 years or older.
The initially increased recall rate after the modality transition exceeded Australian breast screening standards — fewer than 10% of women aged 50–69 years attending their first screening episode are recalled for assessment10 — but declined with time. Nevertheless, the increase could have had a substantial impact on the wellbeing of women who were recalled.29,30 Increased recall rates after the transition to digital mammography in other countries also declined after an adjustment period.31,32 The crude screen‐detected cancer rates for NSW (digital mammography, 6.11 per 1000 screens; film mammography, 4.86 per 1000 screens) were in the middle of the range of values reported overseas, while the crude interval cancer detection rates (digital mammography, 2.84 per 1000 screens; film mammography, 2.56 per 1000 screens) corresponded to the high end of overseas estimates;8 the reason for this difference requires further investigation. We have described the tumour characteristics of screen‐detected and interval cancers in our cohort elsewhere.33
We report the first interrupted time series analysis of the impact on breast screening program outcomes of the transition from film to digital mammography. This approach facilitates assessment of the effectiveness of population‐level health interventions after adjusting for time‐dependent confounders.11 Confounders that change relatively slowly over time, such as population age distribution and obesity, are taken into account by the long term temporal trend estimate.34 We could separately estimate changes in screen‐detected and interval cancer rates attributable to secular population changes and to the transition in screening modality. Our study is also the first to compare film and digital mammography outcomes at the population level in Australia, apart from one case–control study that could not estimate population rates.35 Our analysis of linked statewide data for histologically verified outcomes facilitated a robust analysis.
Limitations
We excluded from our analysis a substantial number of computed radiography screens, screens for which the modality was unknown, and otherwise eligible screens for which interval cancer follow‐up was incomplete. These exclusions reduced the number of included screens undertaken during 2005–2012, and also meant that the proportion of included screens of women aged 40–49 years was smaller for digital than film screens (Supporting Information, table 2). As age influenced screening outcomes by modality, we may have underestimated increases in recall and screen‐detected cancer rates associated with the transition to digital mammography. Our analysis of administrative data was limited by the data collected and recorded; it would be desirable to stratify screening outcomes by mammographic density, but it is not assessed by BreastScreen NSW. Confounders that changed rapidly at the same time as the transition in screening technology may not have been accounted for in our analysis. Nonetheless, population‐based health care data sources provide opportunities for relatively rapid and cost‐efficient quasi‐experimental evaluation and outcomes research. Finally, screen‐detected and interval cancer rates have not been robustly validated as surrogate outcome measures of breast cancer mortality.36
Conclusion
Both screen‐detected cancer and interval cancer rates increased with the move by BreastScreen NSW from film to digital mammography. Adjusting for the background rates reduced the increase in the screen‐detected cancer rate but not that of interval cancer detection. The health benefits of the screening modality transition may have been smaller than anticipated, and were accompanied by increased recall and false positive finding rates, and possibly by overdiagnosis. The effects of future changes in mammography technology, including the introduction of breast tomosynthesis, should be rigorously evaluated.
Box 1 – Study cohort derivation: women aged 40 years or older who were diagnosed with breast cancer (ductal carcinoma in situ or invasive) notified to the NSW Central Cancer Registry and screened by BreastScreen NSW during 1 January 1988 – 31 December 2017
Box 2 – BreastScreen NSW screening (initial and subsequent screens) using film or digital mammography, 2002–2016, by calendar year*
|
Year |
Film mammography |
Digital mammography |
Film and digital mammography |
||||||||||||
|
|
|||||||||||||||
|
2002 |
286 853 |
0 |
286 853 |
||||||||||||
|
2003 |
282 419 |
0 |
282 419 |
||||||||||||
|
2004 |
263 450 |
0 |
263 450 |
||||||||||||
|
2005 |
165 178 |
0 |
165 178 |
||||||||||||
|
2006 |
172 003 (95.7%) |
7687 (4.3%) |
179 690 |
||||||||||||
|
2007 |
159 785 (95.4%) |
7792 (4.7%) |
167 577 |
||||||||||||
|
2008 |
135 815 (84.4%) |
25 163 (15.6%) |
160 978 |
||||||||||||
|
2009 |
50 172 (38.6%) |
79 904 (61.4%) |
130 076 |
||||||||||||
|
2010 |
19 509 (15.8%) |
104 167 (84.2%) |
123 676 |
||||||||||||
|
2011 |
0 |
122 897 |
122 897 |
||||||||||||
|
2012 |
0 |
160 584 |
160 584 |
||||||||||||
|
2013 |
0 |
202 267 |
202 267 |
||||||||||||
|
2014 |
0 |
220 073 |
220 073 |
||||||||||||
|
2015 |
0 |
274 632 |
274 632 |
||||||||||||
|
2016* |
0 |
1205 |
1205 |
||||||||||||
|
Total |
1 535 184 |
1 206 371 |
2 741 555 |
||||||||||||
|
|
|||||||||||||||
|
* Number of screens during 2016 with sufficient follow‐up time for interval cancer detection. |
|||||||||||||||
Box 3 – BreastScreen NSW screening outcomes, by modality (digital, 2006–2016; film mammography, 2002–2010) and screen type (initial or subsequent): unadjusted logistic regression analysis
|
|
Digital mammography |
Film mammography |
Difference |
||||||||||||
|
Outcome |
Number |
Rate per 1000 screens (95% CI) |
Number |
Rate per 1000 screens (95% CI) |
Rate per 1000 screens (95% CI) |
||||||||||
|
|
|||||||||||||||
|
Screens |
1 206 371 |
— |
1 535 184 |
— |
— |
||||||||||
|
Initial screen |
167 339 |
— |
218 492 |
— |
— |
||||||||||
|
Subsequent screen |
1 039 032 |
— |
1 316 692 |
— |
— |
||||||||||
|
Screen‐detected cancer |
7366 |
6.11 (5.97–6.24) |
7468 |
4.86 (4.75–4.97) |
1.24 (1.06 to 1.41) |
||||||||||
|
Initial screen |
1496 |
8.94 (8.49–9.39) |
1421 |
6.50 (6.17–6.84) |
2.43 (1.87 to 2.99) |
||||||||||
|
Subsequent screen |
5870 |
5.65 (5.51–5.79) |
6047 |
4.59 (4.48–4.71) |
1.05 (0.87 to 1.24) |
||||||||||
|
Ductal carcinoma in situ |
1581 |
1.31 (1.24–1.37) |
1196 |
0.77 (0.73–0.82) |
0.53 (0.45 to 0.60) |
||||||||||
|
Initial screen |
315 |
1.88 (1.67–2.09) |
227 |
1.03 (0.90–1.17) |
0.84 (0.59 to 1.09) |
||||||||||
|
Subsequent screen |
1266 |
1.21 (1.15–1.28) |
969 |
0.73 (0.68–0.78) |
0.48 (0.40 to 0.56) |
||||||||||
|
Invasive |
5785 |
4.79 (4.67–4.91) |
6272 |
4.08 (3.98–4.18) |
0.70 (0.55 to 0.86) |
||||||||||
|
Initial screen |
1181 |
7.05 (6.65–7.45) |
1194 |
5.46 (5.15–5.77) |
1.59 (1.08 to 2.09) |
||||||||||
|
Subsequent screen |
4604 |
4.43 (4.30–4.55) |
5078 |
3.85 (3.75–3.96) |
0.57 (0.40 to 0.74) |
||||||||||
|
Interval cancer |
3429 |
2.84 (2.75–2.94) |
3937 |
2.56 (2.48–2.64) |
0.27 (0.15 to 0.40) |
||||||||||
|
Initial screen |
392 |
2.34 (2.11–2.57) |
504 |
2.31 (2.11–2.51) |
0.03 (–0.27 to 0.34) |
||||||||||
|
Subsequent screen |
3037 |
2.92 (2.82–3.03) |
3433 |
2.61 (2.52–2.69) |
0.31 (0.18 to 0.45) |
||||||||||
|
Ductal carcinoma in situ |
345 |
0.28 (0.25–0.31) |
291 |
0.18 (0.16–0.21) |
0.09 (0.05 to 0.13) |
||||||||||
|
Initial screen |
42 |
0.25 (0.17–0.32) |
30 |
0.13 (0.08–0.18) |
0.11 (0.02 to 0.20) |
||||||||||
|
Subsequent screen |
303 |
0.29 (0.25–0.32) |
261 |
0.19 (0.17–0.22) |
0.09 (0.05 to 0.13) |
||||||||||
|
Invasive cancer |
3084 |
2.55 (2.46–2.64) |
3646 |
2.37 (2.29–2.45) |
0.18 (0.06 to 0.29) |
||||||||||
|
Initial screen |
350 |
2.09 (1.87–2.31) |
474 |
2.16 (1.97–2.36) |
–0.07 (–0.37 to 0.21) |
||||||||||
|
Subsequent screen |
2734 |
2.63 (2.53–2.72) |
3172 |
2.40 (2.32–2.49) |
0.22 (0.09 to 0.35) |
||||||||||
|
Recalls |
72 152 |
59.8 (59.4–60.2) |
90 018 |
58.6 (58.3–59.0) |
1.17 (0.60 to 1.73) |
||||||||||
|
Initial screen |
21 709 |
129.7 (128.1–131.3) |
22 358 |
102.3 (101.1–103.6) |
27.4 (25.4 to 29.4) |
||||||||||
|
Subsequent screen |
50 443 |
48.6 (48.1–49.0) |
67 660 |
51.4 (51.0–51.8) |
–2.83 (–3.39 to –2.27) |
||||||||||
|
False positive findings |
63 511 |
52.6 (52.2–53.0) |
80 762 |
52.6 (52.2–53.0) |
0.03 (–0.49 to 0.57) |
||||||||||
|
Initial screen |
20 089 |
120.0 (118.5–121.6) |
20 663 |
94.6 (93.3–95.8) |
25.5 (23.5 to 27.5) |
||||||||||
|
Subsequent screen |
43 422 |
41.8 (41.4–42.2) |
60 099 |
45.6 (45.3–46.0) |
–3.85 (–4.37 to –3.32) |
||||||||||
|
|
|||||||||||||||
|
CI = 95% confidence interval. |
|||||||||||||||
Box 4 – BreastScreen NSW screening outcomes, 2002–2015, by period and screen type (initial or subsequent): interrupted time series analysis*
|
Outcome |
Baseline rate (β0), per 1000 screens |
Change in rate, 2002–2009 (β1), per 1000 screens/year |
Immediate change in rate (β2), per 1000 screens |
Change in rate, 2009–2015 (β3), per 1000 screens/year |
|||||||||||
|
|
|||||||||||||||
|
Screen‐detected cancers |
4.65 |
0.05 |
0.07 |
0.18 |
|||||||||||
|
Initial screen |
5.61 |
0.24 |
0.28 |
0.06 |
|||||||||||
|
Subsequent screen |
4.48 |
0.02 |
0.04 |
0.19 |
|||||||||||
|
Ductal carcinoma in situ |
0.67 |
0.03 |
0.28 |
0.01 |
|||||||||||
|
Initial screen |
0.94 |
0.02 |
0.08 |
0.14 |
|||||||||||
|
Subsequent screen |
0.62 |
0.03 |
0.30 |
–0.01 |
|||||||||||
|
Invasive cancers |
3.98 |
0.02 |
–0.21 |
0.16 |
|||||||||||
|
Initial screen |
4.67 |
0.22 |
0.20 |
–0.08 |
|||||||||||
|
Subsequent screen |
0.62 |
0.03 |
0.30 |
–0.01 |
|||||||||||
|
Interval cancers |
2.52 |
0.01 |
0.75 |
–0.13 |
|||||||||||
|
Initial screen |
1.89 |
0.11 |
–0.25 |
–0.13 |
|||||||||||
|
Subsequent screen |
2.63 |
–0.01 |
0.90 |
–0.13 |
|||||||||||
|
Ductal carcinoma in situ |
0.17 |
0.01 |
0.08 |
0.00 |
|||||||||||
|
Initial screen |
0.11 |
0.01 |
0.11 |
–0.01 |
|||||||||||
|
Subsequent screen |
0.18 |
0.01 |
0.09 |
–0.01 |
|||||||||||
|
Invasive |
2.35 |
0.01 |
0.69 |
–0.13 |
|||||||||||
|
Initial screen |
1.78 |
0.11 |
–0.26 |
–0.14 |
|||||||||||
|
Subsequent screen |
1.88 |
0.08 |
0.02 |
–0.16 |
|||||||||||
|
Recalls |
55.6 |
0.78 |
8.02 |
–2.97 |
|||||||||||
|
Initial screen |
83.7 |
5.25 |
31.0 |
–14.2 |
|||||||||||
|
Subsequent screen |
49.8 |
0.47 |
17.1 |
–6.34 |
|||||||||||
|
False positive results |
49.8 |
0.73 |
7.16 |
–2.95 |
|||||||||||
|
Initial screen |
79.0 |
4.19 |
1.59 |
–2.35 |
|||||||||||
|
Subsequent screen |
44.7 |
0.24 |
8.27 |
–3.15 |
|||||||||||
|
|
|||||||||||||||
|
* Cancer detection rates by time derived from independent models for initial and subsequent screens by cancer type (screen‐detected or interval cancer) are provided in Box 5, and for screen‐detected invasive cancer and ductal carcinomas in situ in Box 6. Recall and false positive rates by time derived from independent models for initial and subsequent screens are provided in Box 7. |
|||||||||||||||
Box 5 – BreastScreen NSW screening outcomes, 2002–2015, by cancer type (screen‐detected or interval cancer) and screen type (initial or subsequent)*
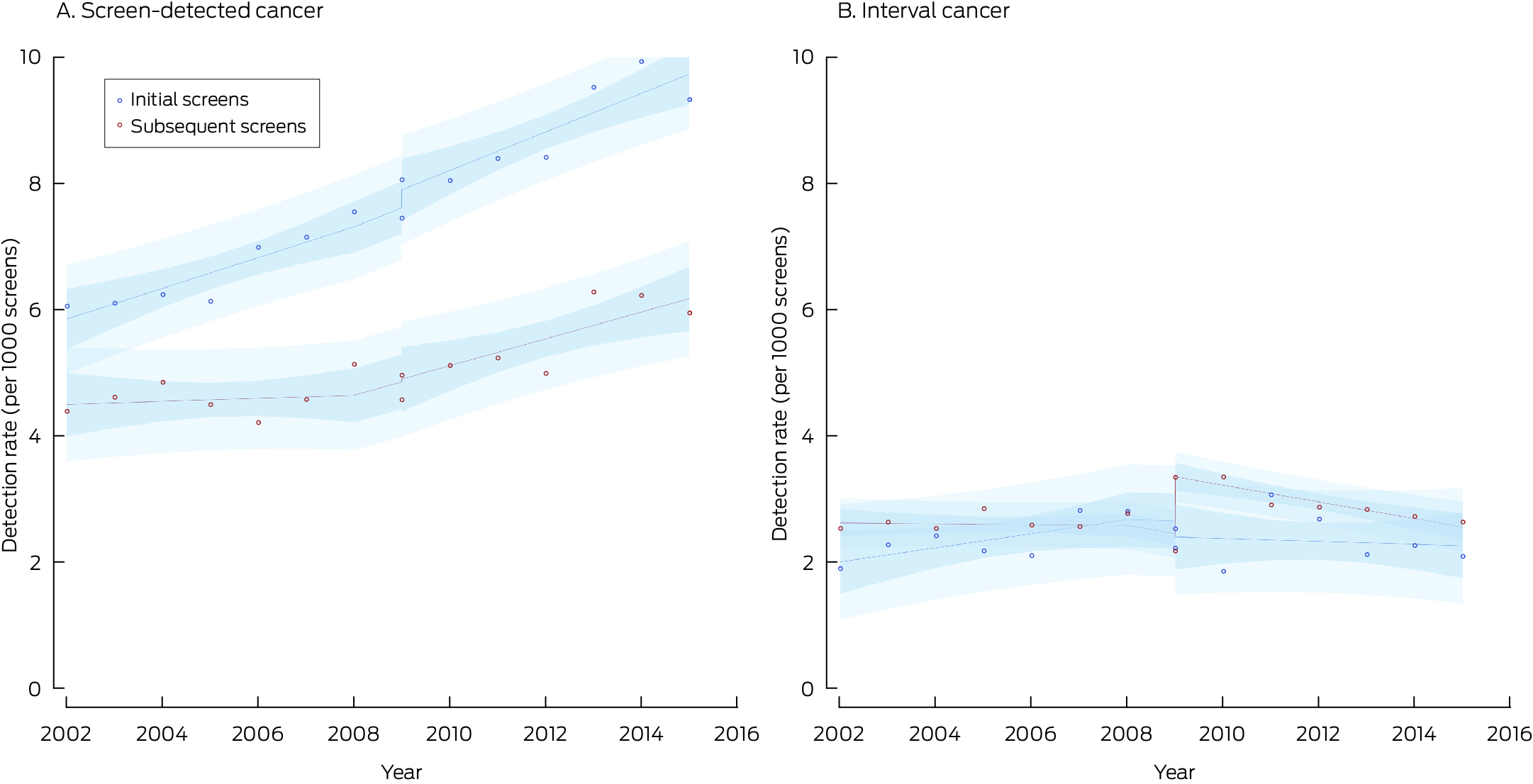
* Points indicate actual values, with fitted curves as dashed lines; dark blue region indicates 95% confidence interval for predicted trend, light blue regions 95% confidence interval for predicted values. Data for 2016 were not included, as the numbers of screens during 2016 with sufficient follow‐up time for interval cancer detection were small.
Box 6 – BreastScreen NSW screen‐detected cancers, 2002–2015, by cancer type (ductal carcinoma in situ or invasive cancer) and screen type (initial or subsequent)*
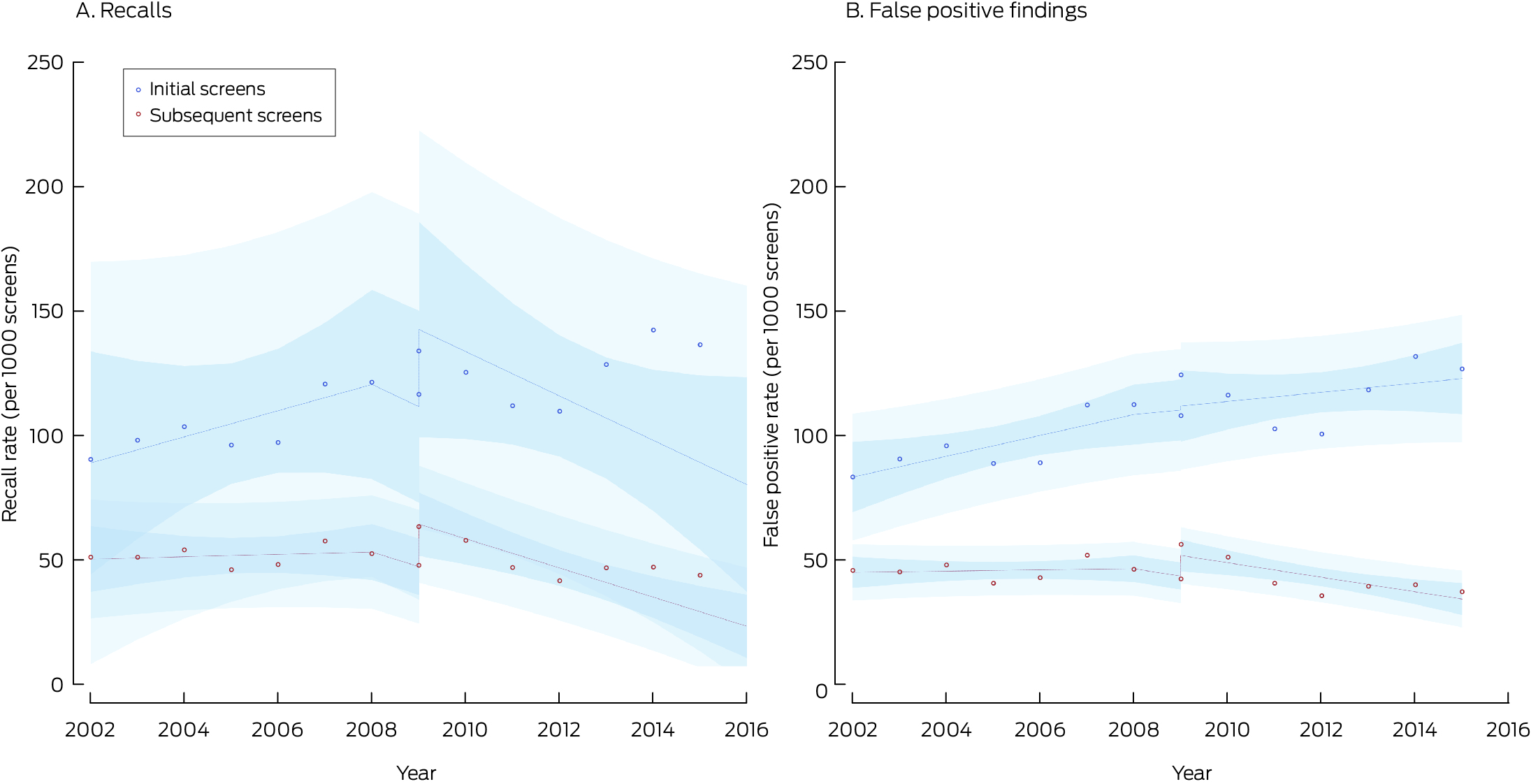
* Points indicate actual values, with fitted curves as dashed lines; dark blue region indicates 95% confidence interval for predicted trend, light blue regions 95% confidence interval for predicted values. Data for 2016 were not included, as the numbers of screens during 2016 with sufficient follow‐up time for interval cancer detection were small.
Box 7 – BreastScreen NSW recall and false positive finding rates, 2002–2015, by screen type (initial or subsequent)*
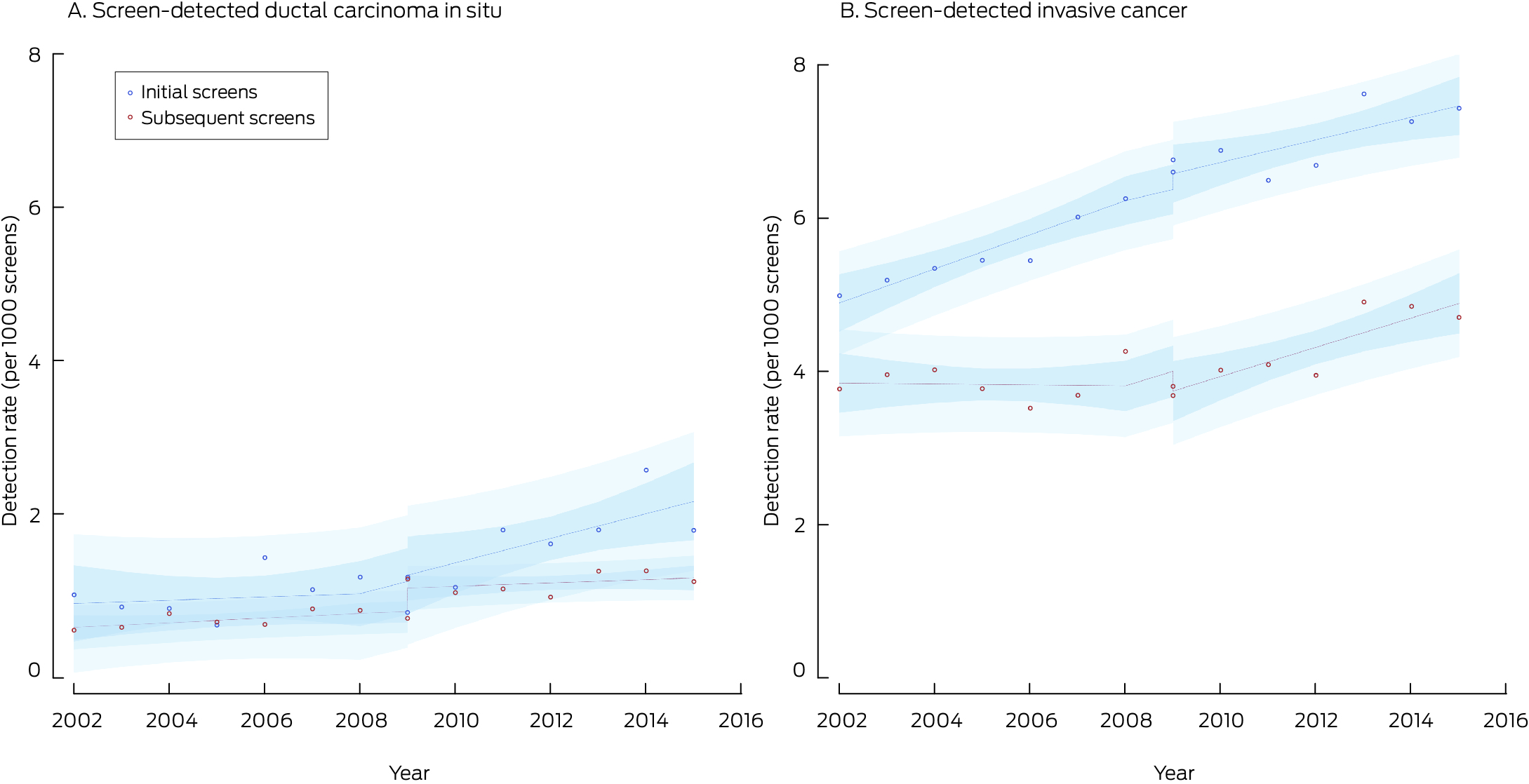
* Points indicate actual values, with fitted curves as dashed lines; dark blue region indicates 95% confidence interval for predicted trend, light blue regions 95% confidence interval for predicted values. Data for 2016 were not included, as the numbers of screens during 2016 with sufficient follow‐up time for interval cancer detection were small.
Received 5 July 2023, accepted 28 March 2024
- Rachel Farber1
- Nehmat Houssami1
- Kevin McGeechan2
- Alexandra L Barratt1
- Katy JL Bell1
- 1 Sydney School of Public Health, the University of Sydney, Sydney, NSW
- 2 The Family Planning Australia Research Centre, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Data Sharing:
Access to the data and analysis files underlying this report is permitted only with the explicit permission of the approving human research ethics committees and the data custodians. Analysis of linked data is currently authorised at only one location.
This study was supported by funding from the National Health and Medical Research Council (NHMRC) Centre for Research Excellence (CIA Alexandra Barratt, 1104136). Rachel Farber received funding from the NHMRC (1168688) and the National Breast Cancer Foundation (NBCF) (DS‐19‐02). Katy Bell holds an NHMRC Investigator grant (1174523). Nehmat Houssami holds an NHMRC Investigator grant (1194410) and an NBCF Chair in Breast Cancer Prevention grant (EC‐21‐001). The funders had no role in the study design, data collection, analysis or interpretation, reporting or publication.
No relevant disclosures.
- 1. Colin C, Vergnon P, Guibaud L, et al. Comparative assessment of digital and analog radiography: diagnostic accuracy, cost analysis and quality of care. Eur J Radiol 1998; 26: 226‐234.
- 2. Wideman C, Gallet J. Analog to digital workflow improvement: a quantitative study. J Digit Imaging 2006; 19 (Suppl 1): 29‐34.
- 3. Pisano ED, Gatsonis C, Hendrick E, et al; Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast‐cancer screening. N Engl J Med 2005; 353: 1773‐1783.
- 4. Skaane P, Hofvind S, Skjennald A. Randomized trial of screen‐film versus full‐field digital mammography with soft‐copy reading in population‐based screening program: follow‐up and final results of Oslo II study. Radiology 2007; 244: 708‐717.
- 5. Vigeland E, Klaasen H, Klingen TA, et al. Full‐field digital mammography compared to screen film mammography in the prevalent round of a population‐based screening programme: the Vestfold County Study. Eur Radiol 2008; 18: 183‐191.
- 6. Karssemeijer N, Bluekens AM, Beijerinck D, et al. Breast cancer screening results 5 years after introduction of digital mammography in a population‐based screening program. Radiology 2009; 253: 353‐358.
- 7. Hambly NM, McNicholas MM, Phelan N, et al. Comparison of digital mammography and screen‐film mammography in breast cancer screening: a review in the Irish breast screening program. AJR Am J Roentgenol 2009; 193: 1010‐1018.
- 8. Farber R, Houssami N, Wortley S, et al. Impact of full‐field digital mammography versus film‐screen mammography in population screening: a meta‐analysis. J Natl Cancer Inst 2021; 113: 16‐26.
- 9. Porter AJ, Evans EB, Erzetich LM. Full‐field digital mammography: is the apparent increased detection of microcalcification leading to over‐investigation and over‐diagnosis? J Med Imaging Radiat Oncol 2017; 61: 470‐475.
- 10. Australian Department of Health and Aged Care. BreastScreen Australia National Accreditation Standards (NAS). Updated 28 Apr 2023. https://www.health.gov.au/resources/publications/breastscreen‐australia‐national‐accreditation‐standards‐nas?language=en (viewed Dec 2023).
- 11. Bazo‐Alvarez JC, Morris TP, Carpenter JR, Petersen I. Current practices in missing data handling for interrupted time series studies performed on individual‐level data: a scoping review in health research. Clin Epidemiol 2021; 13: 603‐613.
- 12. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013; 13: S38‐S44.
- 13. Farber R, Houssami N, McGeechan K, et al. Cohort profile: a data linkage cohort to compare digital vs screen‐film mammography in breast cancer screening in New South Wales, Australia. OSF, 26 May 2023; updated 21 Nov 2023. https://www.osf.io/5eaj9 (viewed Nov 2024).
- 14. HealthStats NSW (NSW Ministry of Health). Population estimates NSW. https://www.healthstats.nsw.gov.au/#/r/111172 (viewed Nov 2024).
- 15. Irvine K, Hall R, Taylor L. A profile of the Centre for Health Record Linkage. Int J Popul Data Sci 2019; 4: 1142.
- 16. Australian Department of Health; BreastScreen Australia. Computed radiography (CR) mammography compared with screen film mammography and digital radiography (DR) mammography. 17 May 2017. https://www.health.gov.au/resources/publications/computed‐radiography‐cr‐mammography‐compared‐with‐screen‐film‐mammography‐and‐digital‐radiography‐dr‐mammography?language=en (viewed Dec 2023).
- 17. Chiarelli AM, Edwards SA, Sheppard AJ, et al; Breast Screening Study Group. Favourable prognostic factors of subsequent screen‐detected breast cancers among women aged 50–69. Eur J Cancer Prev 2012; 21: 499‐506.
- 18. Evans AJ, Pinder SE, Ellis IO, Wilson AR. Screen detected ductal carcinoma in situ (DCIS): overdiagnosis or an obligate precursor of invasive disease? J Med Screen 2001; 8: 149‐151.
- 19. Lagios MD. Heterogeneity of duct carcinoma in situ (DCIS): relationship of grade and subtype analysis to local recurrence and risk of invasive transformation. Cancer Lett 1995; 90: 97‐102.
- 20. Linden A. Conducting interrupted time‐series analysis for single‐ and multiple‐group comparisons. Stata J 2015; 15: 480‐500.
- 21. Jandoc R, Burden AM, Mamdani M, et al. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol 2015; 68: 950‐956.
- 22. Ramsay CR, Matowe L, Grilli R, et al. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 2003; 19: 613‐623.
- 23. Jiang M, Hughes DR, Duszak R. Screening mammography rates in the Medicare population before and after the 2009 US Preventive Services Task Force Guideline Change: an interrupted time series analysis. Womens Health Issues 2015; 25: 239‐245.
- 24. Lopez Bernal J, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol 2018; 47: 2082‐2093.
- 25. Berns EA, Hendrick RE, Solari M, et al. Digital and screen‐film mammography: comparison of image acquisition and interpretation times. Am J Roentgenol 2006; 187: 38‐41.
- 26. Bassett LW. Digital and computer‐aided mammography. Breast J 2000; 6: 291‐293.
- 27. Bick U, Diekmann F. Digital mammography: what do we and what don't we know? Eur Radiol 17: 1931‐1942.
- 28. Ayyala RS, Chorlton M, Behrman RH, et al. Digital mammographic artifacts on full‐field systems: what are they and how do I fix them? RadioGraphics 2008; 28: 1999‐2008.
- 29. Brett J, Austoker J, Ong G. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi‐centre follow‐up study comparing different breast screening result groups five months after their last breast screening appointment. J Public Health Med 1998; 20: 396‐403.
- 30. Brett J, Austoker J. Women who are recalled for further investigation for breast screening: psychological consequences 3 years after recall and factors affecting re‐attendance. J Public Health Med 2001; 23: 292‐300.
- 31. Campari C, Giorgi Rossi P, Mori CA, et al. Impact of the introduction of digital mammography in an organized screening program on the recall and detection rate. J Digit Imaging 2016; 29: 235‐242.
- 32. Bluekens AM, Karssemeijer N, Beijerinck D, et al. Consequences of digital mammography in population‐based breast cancer screening: initial changes and long‐term impact on referral rates. Eur Radiol 2010; 20: 2067‐2073.
- 33. Farber R, Houssami N, McGeechan K, et al. Breast cancer stage and size detected with film versus digital mammography in New South Wales, Australia: a population‐based study using routinely collected data. Cancer Epidemiol Biomarkers Prev 2024; 33: 671‐680.
- 34. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2016; 46: 348‐355.
- 35. Boyle T, Reintals M, Holmes A, et al. Interval cancers as related to frequency of recall to assessment in the South Australian population‐based breast screening program: an exploratory study. Cancer Epidemiol 2022; 79: 102183.
- 36. Jatoi I, Pinsky PF. Breast cancer screening trials: endpoints and overdiagnosis. J Natl Cancer Inst 2021; 113: 1131‐1135.






Abstract
Objectives: To assess the impact of the transition from film to digital mammography in the Australian national breast cancer screening program.
Study design: Retrospective linked population health data analysis (New South Wales Central Cancer Registry, BreastScreen NSW); interrupted time series analysis.
Setting: New South Wales, 2002–2016.
Participants: Women aged 40 years or older with breast cancer diagnosed during 2002–2017 who had been screened by BreastScreen NSW and for whom complete follow‐up information until the end of the recommended re‐screening interval was available.
Intervention: Transition from film to digital mammography; 2009 defined as transition year (digital mammography becomes dominant screening modality).
Main outcome measures: Population rates of screen‐detected cancer, interval cancer, recalls, and false positive findings.
Results: The study cohort comprised 967 573 women; of the 2 741 555 screens, 1 535 184 used film mammography (2002–2010) and 1 206 371 used digital mammography (2006–2016). The screen‐detected cancer rate was 4.86 (95% confidence interval [CI], 4.75–4.97) cases per 1000 screens with film mammography and 6.11 (95% CI, 5.97–6.24) cases per 1000 screens with digital mammography (unadjusted difference, 1.24 [95% CI, 1.06–1.41] cases per 1000 screens). The interval cancer rate was 2.56 (95% CI, 2.48–2.64) cases per 1000 screens with film mammography and 2.84 (95% CI, 2.75–2.94) cases per 1000 screens with digital mammography (unadjusted difference, 0.27 [95% CI, 0.15–0.40] cases per 1000 screens). With the transition to digital mammography, the screen‐detected cancer rate increased by 0.07 per 1000 screens, the sum of the decline in the invasive cancer rate (–0.21 cases per 1000 screens) and the rise in the ductal carcinoma in situ detection rate (0.28 cases per 1000 screens); during 2009–2015, it increased by 0.18 cases per 1000 screens per year. With the transition to digital mammography, the interval cancer rate increased by 0.75 cases per 1000 screens (invasive cancer: by 0.69 cases per 1000 screens); during 2009–2015, it declined by 0.13 cases per 1000 screens per year. The recall rate increased by 8.02 per 1000 screens and the false positive rate by 7.16 per 1000 screens following the transition; both rates subsequently declined to pre‐transition levels.
Conclusions: The increased screen‐detected cancer rate following the transition to digital mammography was not accompanied by a reduction in interval cancer detection rates.