The known: The prospect of healthy ageing is greater for older people with greater intrinsic capacity and an environment supportive of their needs.
The new: The proportion of Aboriginal people aged 45 years or older living in the Kimberley with full intrinsic capacity was 16% at baseline. Intrinsic capacity was unchanged or improved for 40.5% of participants about six years later. The locomotion and sensory domains were the most frequently impaired intrinsic capacity domains.
The implications: Improving hearing, eyesight, and mobility could increase the likelihood of older Aboriginal people ageing well. Having strong intrinsic capacity helps Elders fulfil their roles as custodians of knowledge, language, and culture.
Aboriginal and Torres Strait Islander Australians are living longer lives.1 By 2051, 27% of Indigenous people in Australia will be aged 45 years or older, up from 21% in 2016.2 However, rates of frailty and other health conditions are high among older Indigenous people,3,4,5 and aged care systems are not adequately meeting the health and wellbeing needs of Elders,6 with consequences for individuals, their families, and their communities. Little is known about ageing well for Indigenous Australians and the factors that influence it.
The concept of healthy or successful ageing emerged more than four decades ago.7 One of the most popular models, introduced by Rowe and Kahn,8,9 comprised three components: avoiding disease and disability; high physical and cognitive function; and engagement with life. The second and third aspects of the definition were especially problematic to operationalise. More recently, the World Health Organization (WHO) proposed a framework that defined healthy ageing as “the process of developing and maintaining the functional ability that enables well‐being in older age.”10 Functional ability (defined by the WHO as the “health‐related attributes that enable people to be and to do what they have reason to value”) is determined by an individual's intrinsic capacity (a composite of their physical and mental capacities), environmental factors, and the interaction between the two.10 For example, having support and access to transport can assist older people whose physical and cognitive health are poor to connect with family activities. The combination of intrinsic capacity and environmental factors therefore determines an individual's functional ability.
Based on an extensive literature review, Cesari and colleagues described an approach for operationalising the intrinsic capacity component of the WHO framework.11 Five domains were found to be pivotal to defining a person's intrinsic capacity: the cognition (eg, memory and problem‐solving ability), locomotion (eg, muscle strength and gait), sensory (eg, vision and hearing), vitality (eg, energy and metabolism), and psychological (eg, mood and emotional vitality) domains.11 The WHO subsequently published tools and guidelines to assist organisations use the framework, including strategies to improve and support intrinsic capacity.12 In the past few years, attempts have been made to apply the framework to various populations,13,14 but not yet to Indigenous peoples.
In the study reported in this article, we applied the WHO intrinsic capacity framework to Aboriginal people living in remote Western Australia by analysing data collected during a study of the prevalence of dementia and other medical conditions undertaken in the Kimberley.
Methods
For our longitudinal study, we undertook a secondary analysis of data collected in two studies. During 15 July 2004 – 17 November 2006, we invited Aboriginal people aged 45 years or older living in the Kimberley region of Western Australia to participate in an observational, population‐based cohort study of the prevalence of dementia and other medical conditions.15 People were eligible to participate if they were resident in one of six remote communities (Ardyaloon, Junjuwa, Looma, Mowanjum, Warmun, Wirrimanu) for at least six months of each year. We also recruited a random sample of one in three eligible people living in the town of Derby. Local community health clinics, Derby Regional Hospital, and Kimberley Aboriginal Medical Services Council provided a list of older Indigenous people in their catchment areas that formed the sampling frame for the study. The locations were selected with assistance from local Aboriginal health and community services because they were representative of the five major language groups of the region.
During 8 February 2011 – 6 June 2013, we conducted a follow‐up study that included people who had participated in the first study, as well as 105 newly recruited participants.16 The new participants are not included in the analysis reported in this article, as data were available for only one time point. The sampling frame for the follow‐up study, developed in consultation with local health clinics and community councils, included people aged 45 years or older who were not acutely unwell.
Procedures
At both study timepoints, a team of Aboriginal and non‐Aboriginal researchers and community research assistants administered a culturally appropriate questionnaire to participants and their carers or family members. If required, interpreters translated the survey questions for participants. The questionnaire included a validated measure of cognitive function (the KICA‐Cog),17 as well as a range of items that assessed medical history and the ability to perform activities of daily living. Participants whose KICA‐Cog scores indicated possible cognitive impairment also received a face‐to‐face clinical review by a consultant specialist. If other abnormal clinical results were noted during data collection, participants were referred to a local community health service.
We assessed five domains of intrinsic capacity, and defined “full capacity” in these domains, as follows:
- cognition: specialist review indicated no major cognitive impairment;
- locomotion: participant and family or carer report no difficulty with walking;
- sensory: participant reports no difficulty with vision or hearing;
- vitality: participant and family or carer report participant is eating properly; and
- psychological/mood: participant and family or carer report participant is happy most of the time (further details: Supporting Information, tables 1 and 2).
Participant and family or carer responses to questions about activities of daily living were also used to determine participants’ functional status. Participants unable to dress or shower were deemed to have a core activity limitation. Death before follow‐up was determined by review of local health clinic records and information provided by community research assistants.
Statistical analysis
Data for categorical items are summarised as numbers and proportions, for continuous variables as means with standard deviations (SDs). The statistical significance of between‐group differences was assessed with Pearson χ2 tests (categorical variables) or Mann–Whitney U tests (continuous variables); tests for trend were performed using Cuzick's method.18 The intrinsic capacity score for each participant at each time point was defined as the number of unimpaired intrinsic capacity domains; preserved intrinsic capacity was defined as being alive at follow‐up and having an intrinsic capacity score that was the same or better than at baseline. We assessed associations with unimpaired intrinsic capacity at baseline and with preserved intrinsic capacity at follow‐up in a series of univariable Poisson regression models with robust error variance,19 including factors measured at baseline as independent variables; we report prevalence ratios (PRs) or risk ratios (RRs) with 95% confidence intervals (CIs). We also assessed associations between intrinsic capacity and risk of death by follow‐up and with having a core activity limitation at baseline or follow‐up in Poisson regression analyses adjusted for age at baseline (or age at follow‐up for a cross‐sectional model at follow‐up); we report adjusted PRs or adjusted RRs with 95% CIs. Age was included in these models as a continuous variable. As the proportion of missing data was very small, we performed complete case analyses. Seventeen participants living in residential care were excluded from analyses of associations with alcohol and tobacco use, as they could not smoke or drink alcohol while in care. Data were analysed in Stata SE 17.0; P < 0.05 was deemed statistically significant.
Ethics approval
We conducted the study in alignment with the principles outlined in the Consolidated criteria for strengthening reporting of health research involving Indigenous peoples (CONSIDER) statement20 and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.21 Approval for the study was obtained from the communities involved, the Kimberley Aboriginal Medical Services Council, Kimberley Aged and Community Services, the Kimberley Aboriginal Health Planning Forum research subcommittee (2021‐017), the human research ethics committee of the University of Western Australia (2022/ET000597), and the Western Australian Aboriginal Health Ethics Committee (HREC1072). All participants provided written informed consent for participation.
Results
Participant characteristics at baseline
In the initial study, 363 of 384 invited people participated (response fraction, 94.5%). Seventy‐four participants lived in Derby, 289 in the six remote communities. After excluding eighteen participants for whom intrinsic capacity domain data were incomplete, data for 345 participants were included in baseline analyses (Box 1). The mean age of participants was 60.2 years (SD, 11.6 years; range, 45–96 years); 152 were men (44.1%) and 193 were women (55.9%). The age distributions of men and women were similar (data not shown). The most frequently reported chronic medical conditions were hypertension (148 respondents, 42.9%) and diabetes (142, 41.2%). A core activity limitation was identified at baseline for 23 participants (6.7%; 95% confidence interval [CI], 4.5–9.8%) (Box 1); the most frequent cause was not being able to shower (19 participants).
Intrinsic capacity at baseline
Intrinsic capacity was unimpaired in all five domains for 55 of 345 participants (15.9%; 95% CI, 12.4–20.2%). Capacity in the vitality domain was unimpaired in 325 respondents (94.2%), in the psychological/mood domain in 318 (92.2%), in the cognition domain in 289 people (83.8%), in the locomotion domain in 174 people (50.4%), and in the sensory domain in 117 people (33.9%) (Box 2). The proportion of men with full capacity in all five domains (32 of 152, 21.1%) was larger than for women (23 of 193, 11.9%; P = 0.021); the difference was largely caused by the difference in the locomotion domain (57.2% v 45.1%; P = 0.025). The proportions of people with unimpaired capacity differed by age group for the domains of locomotion, cognition, and vitality (Box 3) with a trend for a lower proportion of unimpaired capacity with older age for locomotion and cognition (P < 0.001).
Change in intrinsic capacity
Of the 345 baseline participants, 63 were lost to follow‐up or declined to participate in the follow‐up study (Supporting Information, figure 1). A total of 185 participants were followed up after a mean of 6.2 years (SD, 0.7 years; range, 5–7 years). The mean age at baseline of responders (59.0 years; SD, 10.7 years) was higher than that of the 63 non‐responders (54.4 years; SD, 7.8 years), and the proportion who had received formal education smaller (118 of 185, 63.8% v 52 of 63, 83%) (Supporting Information, table 3).
After excluding eight of the 185 follow‐up participants for whom data were missing for one or more intrinsic capacity domains at this second timepoint, 274 participants were included in follow‐up analyses (including 97 known to have died). Sixty‐six participants (24.1%) had lost capacity in one to three domains, and 97 (35.4%) had died (Box 4). There was no change in intrinsic capacity for 69 participants (25.2%); intrinsic capacity had improved in 42 participants (15.3%), 33 of whom (79%) were intact in one additional domain. Preserved intrinsic capacity was therefore noted for 111 participants (40.5%; 95% CI, 34.8–46.5%).
Intrinsic capacity at follow‐up
Of the 177 surviving participants for whom complete data were available, intrinsic capacity was unimpaired in all domains for 37 people (20.9%; 95% CI, 15.5–27.6%). The most frequently unimpaired domains were the psychological/mood (162 participants, 91.5%), vitality (162, 91.5%), and cognition domains (133; 75.1%). The proportion of people unimpaired in the sensory domain (85, 48.0%) was larger than at baseline (33.9%), and the proportion of people unimpaired in the locomotion domain (78, 44.1%) was slightly smaller than at baseline (50.4%) (Box 2). The proportions of men and women with full capacity in all five domains were not significantly different (23.9% v 18.9%; P = 0.42). The only statistically significant difference by age was for the cognition domain (P < 0.001) (Box 3). A significant trend was noted with respect to poorer cognition and older age (P < 0.001). A core activity limitation was identified at follow‐up for 14 participants (7.9%; 95% CI, 4.7–13.0%) (Box 1); the most frequent cause was not being able to shower (13 participants).
Factors associated with intrinsic capacity
Being female (PR, 0.57; 95% CI, 0.35–0.93), and having hypertension (PR, 0.57; 95% CI, 0.34–0.98), pain (PR, 0.44; 95% CI, 0.27–0.73), or a history of falls (PR, 0.30; 95% CI, 0.11–0.80) were inversely associated with having full intrinsic capacity in all five domains at baseline (Box 5). The only factors associated with either no change or an improvement in intrinsic capacity between baseline and follow‐up were increasing age (per year: RR, 0.97; 95% CI, 0.96–0.99), currently chewing tobacco (RR, 0.68; 95% CI, 0.50–0.93), and stroke (RR, 1.44; 95% CI, 1.004–2.06) (Box 6).
Associations with risk of death and having a core activity limitation
The number of unimpaired intrinsic capacity domains at baseline (intrinsic capacity score) was inversely associated with having a core activity limitation at baseline (per domain: adjusted PR, 0.43; 95% CI, 0.34–0.55), with having a core activity limitation at follow‐up (per domain: adjusted RR, 0.62; 95% CI, 0.44–0.88), and with risk of death by follow‐up (per domain: adjusted RR, 0.83; 95% CI, 0.71–0.96). Higher intrinsic capacity score at follow‐up was inversely associated with having a core activity limitation at follow‐up (per domain: adjusted PR, 0.69; 95% CI, 0.49–0.96).
Discussion
We report the first study of the application of the intrinsic capacity component of the WHO healthy ageing framework to an Indigenous people. In our cohort study of older Aboriginal people living in remote Western Australia, we found that the proportion of people with full intrinsic capacity was relatively small (16% at baseline, 21% at follow‐up), even among those aged 45–49 years (13% at baseline). The locomotion and sensory domains were least likely to be unimpaired; the vitality, psychological/mood, and cognition domains were relatively strong. The proportion of women with full intrinsic capacity was smaller than for men at baseline, largely because of differences in the locomotion domain. Hypertension, pain, a history of falls, and chewing tobacco were identified as potentially modifiable risk factors for impaired intrinsic capacity. Baseline intrinsic capacity was preserved at follow‐up for only 40.5% of participants in our study.
We found that lower intrinsic capacity was associated with greater risk of death and with having a core activity limitation. However, it is important to note that intrinsic capacity is only one component of the WHO healthy ageing framework, reflecting only its biomedical aspects; such approaches have been criticised for this reason.22 As noted by the WHO, the “environment may be an even stronger influence on functional ability because it determines whether at any given level of intrinsic capacity we can ultimately do the things that are important to us.”10 The WHO age‐friendly cities framework identifies eight environmental domains important for healthy ageing: outdoor spaces and buildings; transportation; housing; social participation; respect and social inclusion; civic participation and employment; communication and information; and community support and health services.23 The framework provides strategies to enhance support for healthy ageing in each domain. There is considerable overlap between the framework and the Good Spirit, Good Life quality of life tool, which measures external and foundational factors considered important by older Aboriginal people for health and ageing.24,25 We are currently recruiting participants for a third wave of our study, which will include assessments with the Good Spirit, Good Life tool to help operationalise the full WHO framework (ie, intrinsic capacity interacting with environmental factors).
Our findings for the domains of cognition and vitality are broadly similar to those of recent studies of intrinsic capacity in other community‐dwelling populations (Box 7). However, the psychological/mood domain was generally better (92% unimpaired at baseline and follow‐up) than what was seen in other studies, while the locomotion and sensory domains were less strong. Our findings indicate the importance of improving the sensory and locomotion capacity of older Indigenous people. The Aboriginal and Torres Strait Islander Peoples Health Assessment (Medicare Benefits Schedule item 715), which must include eye, ear, and hearing examinations, could help achieve this aim.31 The proportion of Indigenous Australians who had these health checks grew from 10% in 2010–11 to 28% in 2017–18 (38% for those aged 55 years or older), but plateaued from 2017–18 to 2020–21.32 The uptake of Indigenous people‐specific health assessments should be increased, and more accessible services provided. Improving access to audiology services may not only improve sensory intrinsic capacity, but also reduce the risk of cognitive decline.33
Impaired vision is a risk factor for falls, and we found that a history of falls was associated with lower intrinsic capacity. The 2016 National Eye Health Survey found that uncorrected refractive error accounted for 64% of cases of vision impairment in Aboriginal adults aged 40 years or older, and cataracts for 20%.34 Potentially, more than 80% of vision loss in Aboriginal adults could be treatable with improved access to optometry, glasses, and cataract surgery. We found that the proportion of people with unimpaired intrinsic capacity in the sensory domain increased from 34% at baseline to 48% at follow‐up. This was not a survivorship effect, but reflected the larger proportion of people who reported good vision at follow‐up (data not shown). However, we could not determine from the available data whether the use of assistive devices had increased since baseline. Other strategies that may reduce the risk of falls, and thereby improve locomotion intrinsic capacity, include medication reviews, falls risk assessments, and modification of the home enviornment.35 Referral to programs for improving muscle strength and balance could also be helpful. The reported association between stroke and no change or an increase in intrinsic capacity at follow‐up may reflect the natural course of the condition, with health improvement typical in the months following a stroke.
The WHO recently developed the Integrated Care for Older People (ICOPE) guidelines, which provide detailed person‐centred strategies for health and social care workers to screen for, assess, and manage declines in intrinsic capacity, with the aim of increasing the likelihood of ageing well.12 Strategies for enhancing function by use of assistive devices and establishing an enabling environment are also provided. This dual approach underpins the concept that a person with limited intrinsic capacity may still have good functional ability provided appropriate environmental supports are in place.10
Limitations
The large response fractions at baseline and follow‐up, and the assessment of participants with culturally appropriate tools, were strengths in our study. However, the sample size was relatively small, limiting the precision of estimates of associations of factors with intrinsic capacity. Recall and response biases were possible; the face‐to‐face interviews could have contributed to the latter, as some participants may have provided responses deemed more socially desirable. We cannot exclude the possibility of a gendered aspect to this, that men perhaps being less likely to report health problems than women. This could potentially contribute to the difference in intrinsic capacity between men and women that we report. Data with which to evaluate intrinsic capacity were limited, particularly with regard to the vitality domain. A recent working definition suggests that vitality should be considered a physiological state that results from the interaction of multiple physiological systems, reflected in the levels of energy and metabolism, neuromuscular function, and immunological and stress responses.36 Our definition, which took only the malnutrition/nutritional status attributes of energy and metabolism into account, is unlikely to fully capture the complexity of this domain. Attrition may have led us to underestimate the proportion of people who are ageing well, given that the mean baseline age of people who participated at follow‐up was higher than for non‐participants; however, their medical comorbidity levels were similar. Finally, the age of the analysed data may limit the generalisability of our findings.
Conclusion
We found that intrinsic capacity was preserved across a period of about six years for about 40% of Aboriginal people aged 45 years or older living in the remote Kimberley region of Western Australia. The locomotion and sensory domains were least strong. However, reduced capacity in these domains could be highly amenable to treatment by improving detection and the accessibility of services. Measures such as the Aboriginal and Torres Strait Islander Peoples Health Assessment may help in this regard, but the provision of optometry and audiology services, including cataract and middle ear surgery, must also be increased. There is also continued need for action to address the social determinants of health to improve the likelihood of Indigenous people ageing well.
Box 1 – Demographic and clinical characteristics of participants at baseline and follow‐up
|
Characteristic |
Baseline |
Follow‐up |
|||||||||||||
|
|
|||||||||||||||
|
Participants |
345 |
177 |
|||||||||||||
|
Age (years), mean (SD) |
60.2 (11.6) |
65.1 (10.1) |
|||||||||||||
|
Age group (years) |
|
|
|||||||||||||
|
45–49 |
71 (20.6%) |
— |
|||||||||||||
|
50–59 |
119 (34.5%) |
71 (40.1%) |
|||||||||||||
|
60–69 |
67 (19.4%) |
47 (26.6%) |
|||||||||||||
|
70–79 |
63 (18.3%) |
40 (22.6%) |
|||||||||||||
|
80 or older |
25 (7.2%) |
19 (10.7%) |
|||||||||||||
|
Sex |
|
|
|||||||||||||
|
Men |
152 (44.1%) |
71 (40.1%) |
|||||||||||||
|
Women |
193 (55.9%) |
106 (59.9%) |
|||||||||||||
|
Some formal education* |
214 (62.0%) |
116 (65.5%) |
|||||||||||||
|
Medical history† |
|
|
|||||||||||||
|
Pain |
201 (58.3%) |
100 (56.5%) |
|||||||||||||
|
Head injury |
178 (51.6%) |
56 (31.6%) |
|||||||||||||
|
Hypertension |
148 (42.9%) |
73 (41.2%) |
|||||||||||||
|
Diabetes |
142 (41.2%) |
88 (49.7%) |
|||||||||||||
|
Falls |
72 (20.9%) |
46 (26.0%) |
|||||||||||||
|
Urinary incontinence |
61 (17.7%) |
61 (34.5%) |
|||||||||||||
|
Heart problems |
59 (17.1%) |
52 (29.4%) |
|||||||||||||
|
Kidney problems |
51 (14.8%) |
45 (25.4%) |
|||||||||||||
|
Stroke |
37 (10.7%) |
25 (14.1%) |
|||||||||||||
|
Polypharmacy |
45 (13.0%) |
94 (53.1%) |
|||||||||||||
|
Alcohol use‡ |
|
|
|||||||||||||
|
Never drank |
93 (27.0%) |
64 (36.2%) |
|||||||||||||
|
Formerly drank (low use) |
68 (19.7%) |
27 (15.3%) |
|||||||||||||
|
Formerly drank (high use) |
55 (15.9%) |
36 (20.3%) |
|||||||||||||
|
Currently drinks (low use) |
112 (32.5%) |
42 (23.7%) |
|||||||||||||
|
Currently drinks (high use) |
17 (4.9%) |
6 (3.4%) |
|||||||||||||
|
Missing data |
0 |
2 |
|||||||||||||
|
Tobacco smoking§ |
|
|
|||||||||||||
|
Never smoked |
147 (42.6%) |
71 (40.1%) |
|||||||||||||
|
Formerly smoked (low use) |
42 (12.2%) |
35 (19.8%) |
|||||||||||||
|
Formerly smoked (high use) |
34 (9.9%) |
20 (11.3%) |
|||||||||||||
|
Currently smokes (low use) |
67 (19.4%) |
36 (20.3%) |
|||||||||||||
|
Currently smokes (high use) |
55 (15.9%) |
12 (6.8%) |
|||||||||||||
|
Missing data |
0 |
3 |
|||||||||||||
|
Tobacco chewing |
|
|
|||||||||||||
|
Never chewed tobacco |
184 (53.3%) |
98 (55.4%) |
|||||||||||||
|
Formerly chewed tobacco |
22 (6.4%) |
17 (9.6%) |
|||||||||||||
|
Currently chews tobacco |
138 (40.0%) |
62 (35.0%) |
|||||||||||||
|
Missing data |
1 |
0 |
|||||||||||||
|
Core activity limitation |
23 (6.7%) |
14 (7.9%) |
|||||||||||||
|
|
|||||||||||||||
|
SD = standard deviation. * Received some education at primary or secondary school level. † Missing data at baseline: stroke, 3; diabetes, 8; hypertension, 12; heart problems, 3; kidney problems, 7; pain, 3; falls, 3; head injury, 1; urinary incontinence, 0; polypharmacy, 0. Missing data at follow‐up: stroke, 1; diabetes, 8; hypertension, 18; heart problems, 3; kidney problems, 5; pain, 3; falls, 3; head injury, 3; urinary incontinence, 2; polypharmacy, 11. ‡ High use = on a daily basis. § High use = one or more packs per day. |
|||||||||||||||
Box 2 – Proportion of participants with unimpaired capacity in each intrinsic capacity domain, at baseline and at follow‐up*
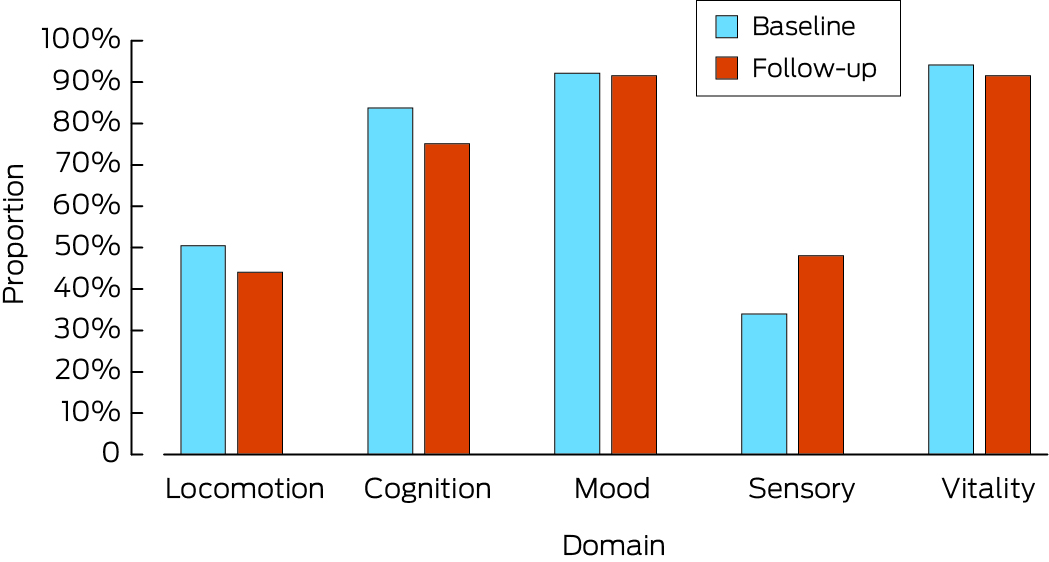
* The data underlying this graph are included in Box 3.
Box 3 – Proportions of participants at baseline and follow‐up with unimpaired intrinsic capacity, overall and by age and sex
|
|
|
Domain |
|
||||||||||||
|
Characteristic |
Participants |
Locomotion |
Cognition |
Psychological/mood |
Sensory |
Vitality |
All domains |
||||||||
|
|
|||||||||||||||
|
Baseline |
|
|
|
|
|
|
|
||||||||
|
All participants |
345 |
174 (50.4%) |
289 (83.8%) |
318 (92.2%) |
117 (33.9%) |
325 (94.2%) |
55 (15.9%) |
||||||||
|
Age group at baseline (years) |
|
|
|
|
|
|
|
||||||||
|
45–49 |
71 |
45 (63%) |
67 (94%) |
64 (90%) |
19 (27%) |
65 (92%) |
9 (13%) |
||||||||
|
50–59 |
119 |
72 (60.5%) |
117 (98.3%) |
110 (92.4%) |
42 (35.3%) |
113 (95.0%) |
25 (21.0%) |
||||||||
|
60–69 |
67 |
33 (49%) |
52 (78%) |
61 (91%) |
26 (39%) |
67 (100%) |
11 (16%) |
||||||||
|
70–79 |
63 |
16 (25%) |
43 (68%) |
59 (94%) |
23 (36%) |
59 (94%) |
8 (13%) |
||||||||
|
80 or older |
25 |
8 (32%) |
10 (40%) |
24 (96%) |
7 (28%) |
21 (84%) |
2 (8%) |
||||||||
|
P* |
— |
< 0.001 |
< 0.001 |
0.87 |
0.56 |
0.041 |
0.34 |
||||||||
|
Sex |
|
|
|
|
|
|
|
||||||||
|
Men |
152 |
87 (57.2%) |
123 (80.9%) |
140 (92.1%) |
56 (36.8%) |
141 (92.8%) |
32 (21.1%) |
||||||||
|
Women |
193 |
87 (45.1%) |
166 (86.0%) |
178 (92.2%) |
61 (31.6%) |
184 (95.3%) |
23 (11.9%) |
||||||||
|
P* |
— |
0.025 |
0.20 |
0.97 |
0.31 |
0.31 |
0.021 |
||||||||
|
Follow‐up |
|
|
|
|
|
|
|
||||||||
|
All participants |
177 |
78 (44.1%) |
133 (75.1%) |
162 (91.5%) |
85 (48.0%) |
162 (91.5%) |
37 (20.9%) |
||||||||
|
Age group at follow‐up (years) |
|
|
|
|
|
|
|
||||||||
|
50–59 |
71 |
36 (51%) |
65 (92%) |
61 (86%) |
36 (51%) |
62 (87%) |
19 (27%) |
||||||||
|
60–69 |
47 |
22 (47%) |
39 (83%) |
46 (98%) |
22 (47%) |
45 (96%) |
11 (23%) |
||||||||
|
70–79 |
40 |
14 (35%) |
23 (58%) |
38 (95%) |
21 (52%) |
38 (95%) |
6 (15%) |
||||||||
|
80 or older |
19 |
6 (32%) |
6 (32%) |
17 (90%) |
6 (32%) |
17 (90%) |
1 (5%) |
||||||||
|
P* |
— |
0.27 |
< 0.001 |
0.11 |
0.46 |
0.33 |
0.15 |
||||||||
|
Sex |
|
|
|
|
|
|
|
||||||||
|
Men |
71 |
37 (52%) |
54 (76%) |
66 (93%) |
37 (52%) |
64 (90%) |
17 (24%) |
||||||||
|
Women |
106 |
41 (38.7%) |
79 (74.5%) |
96 (90.6%) |
48 (45.3%) |
98 (92.5%) |
20 (18.9%) |
||||||||
|
P* |
— |
0.08 |
0.82 |
0.58 |
0.37 |
0.59 |
0.42 |
||||||||
|
|
|||||||||||||||
|
* Pearson χ2 tests. |
|||||||||||||||
Box 4 – Number of unimpaired intrinsic capacity domains for participants at baseline and at follow‐up: Sankey diagram*
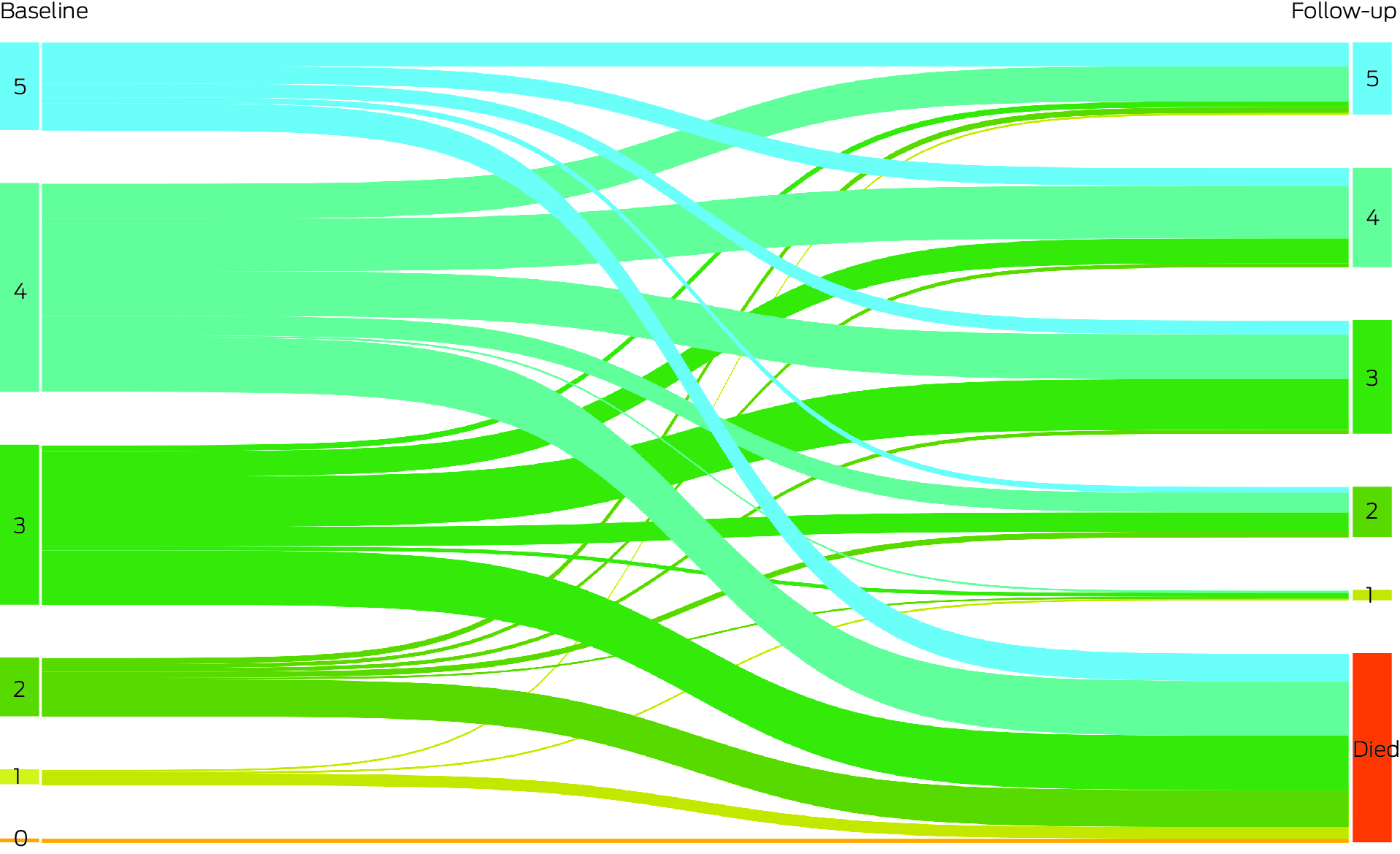
* The data for this graph are included in the Supporting Information, table 4.
Box 5 – Associations of participant characteristics with full intrinsic capacity at baseline: Poisson regression analyses
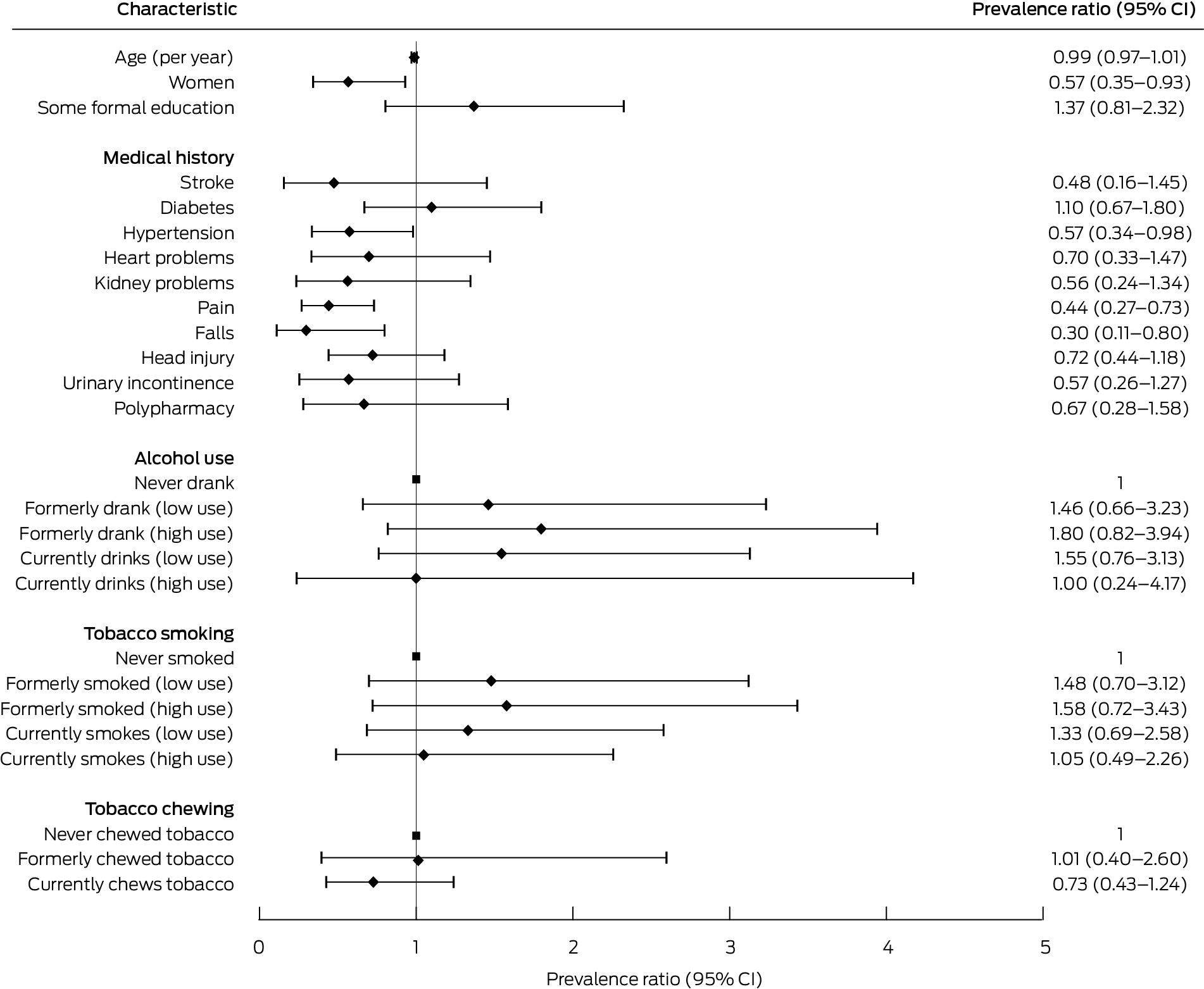
CI = confidence interval.
Box 6 – Associations of participant characteristics with preserved intrinsic capacity at follow‐up:* Poisson regression analyses
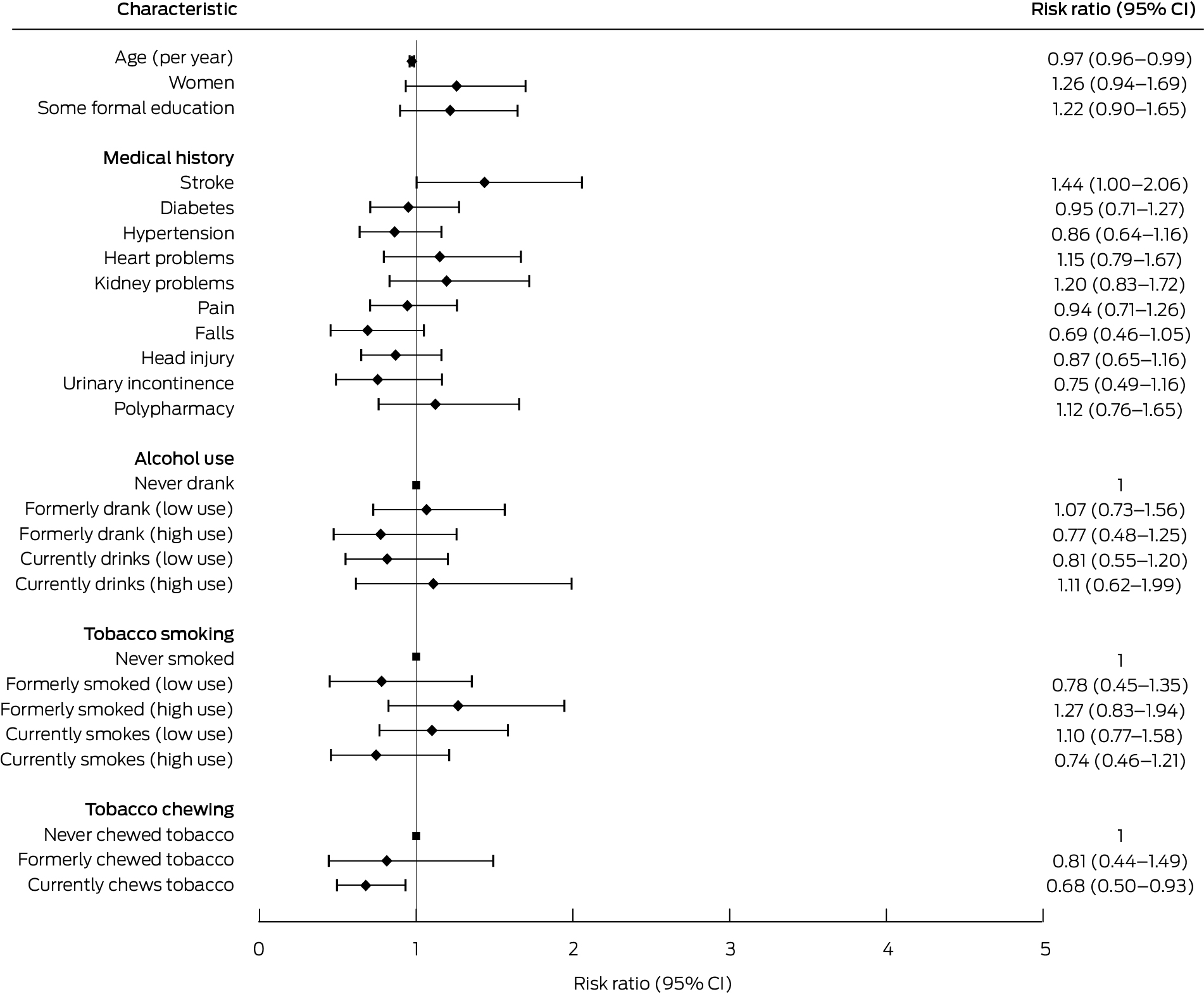
CI = confidence interval.* Number of unimpaired domains same or larger than at baseline.
Box 7 – Prevalence of unimpaired intrinsic capacity reported for people living in the community in other published studies, by domain
|
Characteristic |
Chew et al., 202126 |
Gonzalez‐Bautista et al., 202127 |
Locquet et al., 202228 |
Prince et al., 202129 |
Rarajam Rao et al., 202330 |
||||||||||
|
|
|||||||||||||||
|
Location |
Singapore |
France |
Belgium |
Latin America, India, China |
Southern India |
||||||||||
|
Participants |
200 |
759 |
481 |
Up to 17 031 |
1000 |
||||||||||
|
Age (years) |
Mean, 67.9 (SD, 7.9) |
Mean, 75.2 (SD, 4.3) |
Mean, 73.4 (SD, 6.1) |
Mean (by country), 71.3–76.3 |
Median, 66.5 (IQR, 63–73) |
||||||||||
|
Sex (proportion of women) |
68.5% |
63.6% |
60.1% |
62.4% |
62.9% |
||||||||||
|
Domain |
|
|
|
|
|
||||||||||
|
Locomotion |
67% |
79.8% |
72.8% |
71.2% |
40.7% |
||||||||||
|
Cognition |
75% |
47.8% |
84.0% |
73.5% |
89.4% |
||||||||||
|
Psychological/mood |
86% |
61.0% |
68.0% |
74.1% |
96.2% |
||||||||||
|
Vision |
NA |
81.9% |
NA |
71.3% |
55.9% |
||||||||||
|
Hearing |
NA |
43.8% |
NA |
84.8% |
80.7% |
||||||||||
|
Vitality |
70% |
93.4% |
86.3% |
84.5% |
96.3% |
||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; NA = domain not assessed; SD = standard deviation. |
|||||||||||||||
Received 8 August 2023, accepted 25 March 2024
- Zoë Hyde1,2
- Kate Smith1,2
- Roslyn Malay1
- Dina C LoGiudice3,4
- Dawn C Bessarab2
- David N Atkinson5
- Edward Strivens6,7
- Leon Flicker1
- 1 Western Australian Centre for Health and Ageing, the University of Western Australia, Perth, WA
- 2 Centre for Aboriginal Medical and Dental Health, the University of Western Australia, Perth, WA
- 3 Royal Melbourne Hospital, Melbourne, VIC
- 4 The University of Melbourne, Melbourne, VIC
- 5 Rural Clinical School of Western Australia, the University of Western Australia, Broome, WA
- 6 James Cook University, Cairns, QLD
- 7 Hinterland Hospital and Health Service, Cairns, QLD
Data Sharing:
Data are available from the authors upon reasonable request and after appropriate ethics approval is obtained.
The study was funded by the National Health and Medical Research Council (NHMRC; grants 353612, 634486, 1170422). Leon Flicker holds a Medical Research Future Fund Practitioner Fellowship (grant 1155669). The funders had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
We gratefully acknowledge the assistance of the community members living in Derby, Ardyaloon, Warmun, Wirrimanu, Looma, Junjuwa, and Mowanjum who participated in this project.
No relevant disclosures.
- 1. National Indigenous Australians Agency. Closing the gap. Report 2020. Canberra: Department of the Prime Minister and Cabinet, 2020. https://ctgreport.niaa.gov.au/sites/default/files/pdf/closing‐the‐gap‐report‐2020.pdf (viewed Feb 2024).
- 2. Temple JB, Wilson T, Taylor A, et al. Ageing of the Aboriginal and Torres Strait Islander population: numerical, structural, timing and spatial aspects. Aust N Z J Public Health 2020; 44: 271‐278.
- 3. Hyde Z, Flicker L, Smith K, et al. Prevalence and incidence of frailty in Aboriginal Australians, and associations with mortality and disability. Maturitas 2016; 87: 89‐94.
- 4. Hyde Z, Smith K, Flicker L, et al. HbA1c is associated with frailty in a group of Aboriginal Australians. J Frailty Aging 2019; 8: 17‐20.
- 5. Australian Institute of Health and Welfare. Contribution of chronic disease to the gap in adult mortality between Aboriginal and Torres Strait Islander and other Australians (cat. no. IHW 48). May 2011. https://www.aihw.gov.au/getmedia/79b73a27‐c970‐47f0‐931b‐32d7badade40/12304.pdf (viewed Feb 2024).
- 6. Royal Commission into Aged Care Quality and Safety. Final report: care, dignity and respect. 1 Mar 2021. https://www.royalcommission.gov.au/aged‐care/final‐report (viewed Feb 2024).
- 7. Palmore E. Predictors of successful aging. Gerontologist 1979; 19: 427‐431.
- 8. Rowe JW, Kahn RL. Human aging: usual and successful. Science 1987; 237: 143‐149.
- 9. Rowe JW, Kahn RL. Successful aging. Gerontologist 1997; 37: 433‐440.
- 10. World Health Organization. World report on ageing and health. 2015. https://iris.who.int/bitstream/handle/10665/186463/9789240694811_eng.pdf (viewed Feb 2024).
- 11. Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci 2018; 73: 1653‐1660.
- 12. Integrated care for older people (ICOPE). Guidance for person‐centred assessment and pathways in primary care (WHO/FWC/ALC/19.1). Geneva: World Health Organization, 2019. https://iris.who.int/bitstream/handle/10665/326843/WHO‐FWC‐ALC‐19.1‐eng.pdf (viewed Feb 2024).
- 13. Koivunen K, Hoogendijk EO, Schaap LA, et al. Development and validation of an intrinsic capacity composite score in the Longitudinal Aging Study Amsterdam: a formative approach. Aging Clin Exp Res 2023; 35: 815‐825.
- 14. Aliberti MJR, Bertola L, Szlejf C, et al. Validating intrinsic capacity to measure healthy aging in an upper middle‐income country: findings from the ELSI‐Brazil. Lancet Reg Health Am 2022; 12: 100284.
- 15. Smith K, Flicker L, Lautenschlager NT, et al. High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology 2008; 71: 1470‐1473.
- 16. Lo Giudice D, Smith K, Fenner S, et al. Incidence and predictors of cognitive impairment and dementia in Aboriginal Australians: a follow‐up study of 5 years. Alzheimers Dement 2016; 12: 252‐261.
- 17. LoGiudice D, Smith K, Thomas J, et al. Kimberley Indigenous Cognitive Assessment tool (KICA): development of a cognitive assessment tool for older indigenous Australians. Int Psychogeriatr 2006; 18: 269‐280.
- 18. Cuzick J. A Wilcoxon‐type test for trend. Stat Med 1985; 4: 87‐90.
- 19. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702‐706.
- 20. Huria T, Palmer SC, Pitama S, et al. Consolidated criteria for strengthening reporting of health research involving indigenous peoples: the CONSIDER statement. BMC Med Res Methodol 2019; 19: 173.
- 21. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344‐349.
- 22. Martinson M, Berridge C. Successful aging and its discontents: a systematic review of the social gerontology literature. Gerontologist 2015; 55: 58‐69.
- 23. World Health Organization. Global age‐friendly cities: a guide. 2007. https://iris.who.int/bitstream/handle/10665/43755/9789241547307_eng.pdf (viewed Feb 2024).
- 24. Smith K, Gilchrist L, Taylor K, et al. Good Spirit, Good Life: a quality of life tool and framework for older Aboriginal peoples. Gerontologist 2021; 61: e163‐e172.
- 25. Gilchrist L, Hyde Z, Petersen C, et al. Validation of the Good Spirit, Good Life quality‐of‐life tool for older Aboriginal Australians. Australas J Ageing 2023; 42: 302‐310.
- 26. Chew J, Lim JP, Yew S, et al. Disentangling the relationship between frailty and intrinsic capacity in healthy community‐dwelling older adults: a cluster analysis. J Nutr Health Aging 2021; 25: 1112‐1118.
- 27. Gonzalez‐Bautista E, de Souto Barreto P, Andrieu S, et al. Screening for intrinsic capacity impairments as markers of increased risk of frailty and disability in the context of integrated care for older people: secondary analysis of MAPT. Maturitas 2021; 150: 1‐6.
- 28. Locquet M, Sanchez‐Rodriguez D, Bruyère O, et al. Intrinsic capacity defined using four domains and mortality risk: a 5‐year follow‐up of the SarcoPhAge cohort. J Nutr Health Aging 2022; 26: 23‐29.
- 29. Prince MJ, Acosta D, Guerra M, et al. Intrinsic capacity and its associations with incident dependence and mortality in 10/66 Dementia Research Group studies in Latin America, India, and China: a population‐based cohort study. PLoS Med 2021; 18: e1003097.
- 30. Rarajam Rao A, Waris M, Saini M, et al. Prevalence and factors associated with impairment in intrinsic capacity among community‐dwelling older adults: an observational study from South India. Curr Gerontol Geriatr Res 2023; 2023: 4386415.
- 31. Bailie J, Laycock A, Matthews V, et al. Emerging evidence of the value of health assessments for Aboriginal and Torres Strait Islander people in the primary healthcare setting. Aust J Prim Health 2019; 25: 1‐5.
- 32. Australian Institute of Health and Welfare. Ear and hearing health of Aboriginal and Torres Strait Islander people 2021 (cat. no. IHW 262). 2022. https://www.aihw.gov.au/getmedia/557cc818‐3c84‐4138‐b8a5‐daf1b35f28ba/aihw‐ihw‐262.pdf (viewed Feb 2024).
- 33. Naylor G, Dillard L, Orrell M, et al. Dementia and hearing‐aid use: a two‐way street. Age Ageing 2022; 51: afac266.
- 34. Foreman J, Keel S, Xie J, et al. The National Eye Health Survey 2016. Melbourne: Centre for Eye Research Australia; Vision 2020 Australia, 2016. https://www.vision2020australia.org.au/wp‐content/uploads/2019/06/National‐Eye‐Health‐Survey_Full‐Report_FINAL.pdf (viewed Feb 2024).
- 35. Hill KD, Flicker L, LoGiudice D, et al. Falls risk assessment outcomes and factors associated with falls for older Indigenous Australians. Aust N Z J Public Health 2016; 40: 553‐558.
- 36. Bautmans I, Knoop V, Amuthavalli Thiyagarajan J, et al; WHO Working Group on Vitality Capacity. WHO working definition of vitality capacity for healthy longevity monitoring. Lancet Healthy Longev 2022; 3: e789‐e796.





Abstract
Objective: To assess intrinsic capacity, an important component of ageing well, in older Aboriginal people living in remote Western Australia.
Study design: Longitudinal cohort study; secondary analysis of survey and clinical assessment data.
Setting: Kimberley region of Western Australia (six remote communities, and the town of Derby).
Participants: Aboriginal people aged 45 years or older, initially recruited 15 July 2004 – 17 November 2006.
Main outcome measures: Intrinsic capacity (assessed in each participant by questionnaire and review by a consultant specialist), overall and by domain, and presence of core activity limitations, at baseline and follow‐up (8 February 2011 – 6 June 2013); risk of death by follow‐up; preservation of intrinsic capacity at follow‐up.
Results: The mean age of the 345 participants at baseline was 60.2 years (standard deviation [SD], 11.6 years; range, 45–96 years); 152 were men (44.1%) and 193 were women (55.9%). Intrinsic capacity was unimpaired in all five domains for 55 participants (15.9%; 95% confidence interval [CI], 12.4–20.2%). Capacity in the vitality domain was unimpaired in 325 respondents (94.2%), in the psychological/mood domain in 318 (92.2%), and in the cognition domain in 289 people (83.8%); the locomotion domain was unimpaired in 174 people (50.4%), and the sensory domain in 117 people (33.9%). The proportion of men with full capacity in all five domains (32 of 152, 21.1%) was larger than for women (23 of 193, 11.9%). Of the 274 people included in follow‐up analyses, intrinsic capacity was lower than at baseline for 66 people (24.1%), it was unchanged or improved in 111 participants (40.5%; 95% CI, 34.8–46.5%), and 97 people had died (35.4%). Thirty‐seven of the 177 surviving participants for whom complete data were available had full capacity in all domains (20.9%; 95% CI, 15.5–27.6%). After adjustment for age, the number of unimpaired intrinsic capacity domains at baseline was inversely associated with having a core activity limitation at baseline (per domain: adjusted prevalence ratio, 0.43; 95% CI, 0.34–0.55) and follow‐up (adjusted risk ratio, 0.62; 95% CI, 0.44–0.88), and with risk of death by follow‐up (adjusted risk ratio, 0.83; 95% CI, 0.71–0.96).
Conclusions: Impaired intrinsic capacity in older Aboriginal people living in the Kimberley was most frequent in the sensory and locomotion domains. Reduced capacity in these domains could be highly amenable to treatment that would ensure that Elders can continue to take part in activities important for quality of life.