The known: COVID‐19 vaccines are associated with a slightly increased risk of myocarditis.
The new: In Victoria, COVID‐19 vaccine‐associated myocarditis was generally milder than for other myocarditis forms. Electrocardiographic abnormalities were more frequent in male patients and those aged 24 years or younger; cardiac MRI abnormalities were not seen in patients for whom troponin levels were increased threefold or less.
The implications: Young men are at particular risk of COVID‐19 vaccine‐associated myocarditis, and warrant close follow‐up to determine long term outcomes. A threefold or greater increase in troponin level may be a clinically useful predictor of cardiac MRI abnormalities.
The possibility of post‐vaccination myocarditis was recognised following mass vaccination programs around the world during the coronavirus disease 2019 (COVID‐19) pandemic.1,2,3,4 COVID‐19 vaccine‐associated myocarditis is most frequently diagnosed in young men who have received mRNA‐based vaccines.1,2,3,4 The epidemiology of this adverse event has been described in several countries,1,2,3,4 but only limited information about the clinical phenotype and investigations of affected people is available. As vaccines are likely to remain a core component of the ongoing response to COVID‐19, particularly in the form of seasonal vaccinations, knowledge about this adverse event is important for safely implementing vaccination recommendations.
The Australian COVID‐19 vaccination program began on 22 February 2021.5 The Therapeutic Goods Administration (TGA) initially approved BNT162b2 (Comirnaty, Pfizer–BioNTech) and AZD122 (Vaxzevria, AstraZeneca) for use in Australia, and subsequently mRNA‐1273 (Spikevax, Moderna) and NVX–CoV2373 (Nuvaxovid, Novavax). Vaccination was available to people aged 16 years or older from 19 August 2021, to those aged 12–15 years from 13 September 2021, to children aged 5–11 years from 10 January 2022, and to those aged 6 months to 5 years from 29 September 2022.5
The aim of our study was to describe myocarditis as an adverse event after COVID‐19 vaccination, including a detailed description of clinical phenotypes and diagnostic test results and differences by age, sex, and degree of troponin level elevation.
Methods
For our cross‐sectional study, we initially included all reports to Surveillance of Adverse Events Following Vaccination In the Community (SAEFVIC) of suspected myocarditis following the administration of any COVID‐19 vaccine in Victoria during 22 February 2021 – 30 September 2022. SAEFVIC is the statewide vaccine safety surveillance system, comprising both spontaneous and active surveillance components. Specialist clinicians review reports of suspected adverse post‐vaccination events reported by health care providers or the public; further clinical details may be collated to facilitate appropriate coding.6 For our study, two authors (DRC, NWC) independently reviewed the extracted reports; cases were excluded if symptom onset was more than 14 days after vaccination or an alternative cause was deemed more likely.
Data sources
The diagnostic certainty of reported cases of post‐COVID‐19 vaccine myocarditis was evaluated using the Brighton Collaboration Criteria for myocarditis.7 Cases classified as level 1 (definitive), level 2 (probable), or level 3 (possible) myocarditis were deemed to be confirmed cases of myocarditis and included in our analysis. Cases were categorised by age group (24 years or younger, older than 24 years), in line with Centers for Disease Control and Prevention (United States) reporting.4 We extracted demographic data (age and sex), vaccine details (COVID‐19 vaccine type, dose number), presentation details (time to symptom onset, place of presentation or admission: emergency department, hospital ward, intensive care unit), need for intensive treatment, survival, symptoms at presentation (chest pain, dyspnoea, palpitations, diaphoresis, nausea), and investigation results (blood troponin, electrocardiography, echocardiography, cardiac magnetic resonance imaging [cMRI]). To account for variation in troponin assays used in different laboratories, troponin levels are reported as multiples of the upper limit of normal value at the testing laboratory.
Statistical analysis
Patient data were collected and managed in REDCap8,9 electronic data capture tools hosted by SAEFVIC. We summarise categorical data as numbers and proportions, and non‐parametric continuous data as medians with interquartile ranges (IQRs). The statistical significance of between‐group differences was assessed in Pearson χ2 or Fisher exact tests (categorical variables) or Mann–Whitney U tests (continuous variables). Associations between variables and binary outcomes (eg, symptoms, imaging results) were assessed in logistic regression analyses adjusted for age group and sex; for continuous outcomes (eg, time to symptom onset, troponin level), associations were assessed in linear regression analyses adjusted for age group and sex. We report adjusted odds ratios (aORs) with 95% confidence intervals (CIs). All regression analyses were subsequently repeated with an interaction term for age group and sex; however, as no significant interaction between age group and sex was evident, all reported results are for regression analyses that treated age and sex as independent covariates. Statistical analyses were undertaken in R 4.1.0; (R Foundation for Statistical Computing). P < 0.05 was deemed statistically significant.
Ethics approval
Follow‐up of cases of COVID‐19 vaccine‐associated myocarditis was undertaken as part of the public health response to adverse events following vaccination. Our analysis of SAEFVIC data conformed with its function as a public health vaccine surveillance system, which is approved by the Royal Children's Hospital Health Research Ethics Committee as a registered database (36219).
Results
During 22 February 2021 – 30 September 2022, more than 16.5 million COVID‐19 vaccine doses were administered in Victoria.10 During this period, 454 possible cases of COVID‐19 vaccine‐associated myocarditis were recorded by SAEFVIC. Based on vaccine distribution and population data, we estimated the myocarditis case rate to be 2.1 per 100 000 vaccine doses for dose 1 (all brands) and 5.6 per 100 000 vaccine doses for dose 2.11
We excluded 182 cases from our analysis that did not meet the Brighton Collaboration criteria. A further 40 cases were excluded because the time of symptom onset was more than 14 days after vaccination; one case was excluded because the available details were insufficient. Of the 231 remaining cases, 206 were classed as confirmed cases of myocarditis according to the Brighton Collaboration criteria (level 1, 68 cases; level 2, 138 cases); for 25 cases an alternative cause of myocarditis was identified.
Patient characteristics
The median age of people with confirmed myocarditis was 21 years (IQR, 16–32 years; range, 10–76 years); 129 were aged 24 years or younger (63%), 77 were under eighteen years of age (37%). The median age of the 155 male patients with confirmed myocarditis (19 [IQR, 16–31] years) was lower than for the 51 female patients (24 [IQR, 21–35] years). A total of 201 cases (98%) followed the administration of mRNA vaccines (171 after BNT162b2, 83%; 30 after mRNA–1273, 15%); five cases followed vaccination with AZD122, and none followed NVX‐CoV2373. Forty‐six cases followed first vaccine doses (22%), 138 cases second doses (67%), and 22 cases third vaccine doses (11.0%) (Box 1).
In 201 cases (98%), people initially presented to emergency departments. A total of 129 people were admitted to hospital (63%); their median length of stay was two days (IQR, 1–3 days; range, 0–33 days) (Box 1). Five people were admitted to intensive care; two people required inotropic support and extracorporeal membrane oxygenation (ECMO). The sole reported death was investigated by the TGA Vaccine Safety Investigation Group, which concluded that COVID‐19 vaccination may have contributed to the development of myocarditis, but other significant factors affected the clinical course.12
Symptom onset and symptoms at presentation
The median time from vaccination to symptom onset was two days (IQR, 1–4 days); it was shorter for people aged 24 years or younger (two days; IQR, 1–3 days) than for those over 24 years of age (three days; IQR, 1–6 days; P = 0.004). Time to symptom onset did not differ significantly by sex, vaccine, or dose number (data not shown).
The most frequent symptom on presentation was chest pain (204 people, 99%) (Box 2). Palpitations were less frequently reported by male than female patients (40, 26% v 28, 55%; aOR, 0.77; 95% CI, 0.66–0.89) (Box 3).
Cardiac investigations
Troponin levels were elevated in 205 of 206 patients. The median increase, relative to the local upper limit of normal, was 60.8‐fold (IQR, 5–234‐fold); the median increase was greater in male (95.3‐fold; IQR, 5.8–273‐fold) than female patients (9.9‐fold; IQR, 4.7–50‐fold), and greater for those aged 24 years or younger (108‐fold; IQR, 9.9–273‐fold) than for patients over 24 years of age (10.6‐fold; IQR, 3.1–97.6‐fold) (Box 4). In multivariate analyses, the magnitude of the troponin level increase (≤ 5‐fold v > 5‐fold) was not significantly influenced by age group or sex (Box 3).
Electrocardiography was performed in all 206 patients; abnormal results were recorded for 104 (50.5%) (Box 5). The proportion of female patients with abnormal results was smaller than for male patients (15, 30% v 89, 57.8%; aOR, 0.75; 95% CI, 0.64–0.89). The proportions of people aged 24 years or younger and of those over 24 years of age with abnormal results were similar (70, 54.7% v 34, 44.7%; aOR, 1.07; 95% CI, 0.93–1.24) (Box 3).
Echocardiography was performed in 200 of 206 patients; abnormal results were recorded for 26 (13%) (Box 5). The proportions of male and female patients with abnormal results were similar (22, 14.6% v 4, 8%; aOR, 0.91; 95% CI, 0.80–1.04), as were those of people aged 24 years or younger and of those over 24 years of age (16, 12.8% v 10, 13.3%; aOR, 0.98; 95% CI, 0.87–1.11) (Box 3).
Cardiac MRI was performed in 61 patients (30%); the results were abnormal for 54 (88%) (Box 5). The proportions of male and female patients with abnormal results were similar (46, 92% v 8, 73%; aOR, 0.89; 95% CI, 0.70–1.14), as were those of people aged 24 years or younger and of those over 24 years of age (42, 93% v 12, 75%; aOR, 1.11; 95% CI, 0.90–1.37). The likelihood of abnormal cMRI results was not statistically higher for those with abnormal echocardiography results (Box 3).
Troponin level increase and cardiac MRI findings
The median relative troponin level increase was greater for people with abnormal cMRI findings (298‐fold; IQR, 74.6–648‐fold; range, 2.9–9364‐fold) than for people without cMRI abnormalities (16.9‐fold; IQR, 7.5–63.2‐fold; range, 1–183‐fold) (Box 4). The median troponin level increase was also higher for people with echocardiogram abnormalities (350‐fold; IQR, 75.5–1024‐fold) than for people with normal echocardiograms (51.6; IQR, 5–186‐fold).
Discussion
In this article we have reported the largest case series of COVID‐19 vaccine‐associated myocarditis in Australia, describing its clinical phenotype and course. Our study built on our earlier description of vaccine‐associated myocarditis in 12–17‐year‐old Victorians13 by including people of all ages and covering the entire population COVID‐19 vaccination period.
We found that more cases of myocarditis were associated with BNT162b2 than with mRNA‐1273. This finding probably reflects the greater use of BNT162b2 in Australia during 2021, when it was the major available vaccine. Conversely, the relatively low number of cases associated with AZD122 and NVX‐CoV2373 reflects their lower availability and the fact that fewer doses were given to people at greater risk of COVID‐19 vaccine induced myocarditis.
The median hospital length of stay for people with COVID‐19 vaccine‐associated myocarditis (two days) was similar to that reported by other studies (eg, three days for fifteen cases in Denmark).14 In contrast, the reported mean length of stay for people in the United States with myocarditis (any aetiology) during 2005–2014 was 7.4 days.15 Similarly, the low numbers of intensive care unit admissions and deaths in our study contrast with reports that as many as 54% of cases of myocarditis (any aetiology) lead to intensive care unit admissions,15 and of mortality as high as 6.3%15 or 7.8%.16 COVID‐19 vaccine‐associated myocarditis in Victoria was relatively mild and its initial clinical presentation brief compared with other forms of myocarditis.
Phenotypic characteristics
Three in four cases of COVID‐19 vaccine‐associated myocarditis in our study were in young men, consistent with reports about myocarditis in general.17 Possible biological explanations include the pro‐inflammatory effect of testosterone and age‐related sex hormone profiles, but myocarditis may be underdiagnosed in women because its clinical features are more subtle.18 We found that female patients more frequently presented with non‐classical features, including palpitations and nausea, than male patients.
We found that larger proportions of male patients and people aged 24 years or younger had abnormal cardiac investigation findings; the proportions of patients with elevated troponin levels did not differ significantly by sex or age group after adjusting for other factors. Although troponin level elevation alone is not associated with poorer prognosis for people with myocarditis,19 it may be an important marker of myocyte damage.20
The proportion of male patients with electrocardiographic abnormalities was significantly higher than for female patients. Electrocardiographic changes are not always found in people with myocarditis, but they are associated with longer hospital stays.21
Higher rates of abnormal echocardiography and cMRI findings in male patients and of abnormal cMRI findings in those aged 24 years or younger were not statistically significant. However, only a relatively small number of people underwent cMRI; the number undertaken at the time of diagnosis was limited by funding and resource constraints. As myocarditis can evolve over time, we are undertaking further cMRI evaluations and long term imaging of sequelae in our study cohort.22 This is important because certain echocardiographic abnormalities are associated with poorer outcomes for people with myocarditis,23 and cMRI is an important tool for both diagnosing and predicting adverse outcomes.24
Two different COVID‐19 vaccine‐associated myocarditis phenotypes could be distinguished in our study. For male patients and people aged 24 years or younger, median time to symptom onset was shorter, median troponin level increases were greater, and rates of abnormal electrocardiographic findings were higher. For female patients and people over 24 years of age, median troponin increases were smaller, and rates of abnormal electrocardiographic findings were lower. Further evaluation will determine whether long term outcomes differ by phenotype.
Troponin level thresholds
We found that troponin level elevation was greater in people with abnormal cMRI findings; no cMRI abnormalities were found in patients for whom the troponin increase was threefold or less. This degree of increase could be used during initial assessments as a threshold for identifying people who are at lower risk of developing cMRI abnormalities. Clinically relevant troponin increase thresholds could assist risk stratification, investigation, and follow‐up of people with COVID‐19 vaccine‐associated myocarditis, especially in hospitals with limited access to cMRI facilities.
Limitations
Three‐quarters of the study population were young male patients, but our multivariate analysis assessed the influence of sex and age group. Excluding myocarditis cases linked with other causes could be a source of bias. Further, the available clinical laboratory data for the included cases were limited; for example, a concurrent SARS‐CoV‐2 infection could also cause myocarditis.
Given the nature of surveillance reports submitted to SAEFVIC, the information received may be influenced by biases that affect reporting behaviour. As we undertook a retrospective data analysis, and clinical investigations were performed at the discretion of local treating teams, not all preferred investigations were conducted in every case, which may limit diagnostic certainty in some cases.
Conclusion
We have described the clinical features of COVID‐19 vaccine‐associated myocarditis in Victoria, and found that clinical severity was generally mild. Markers of a more severe myocarditis phenotype were more frequently recorded for male patients and people aged 24 years or younger. However, myocarditis can affect people of any age or sex, and after receiving most COVID‐19 vaccines. A balance between sufficiently cautionary public health information and promoting the broader benefits of vaccination at the population level is needed. A threshold troponin level increase of three or more times the upper limit of normal could be considered when assessing the likelihood of cardiac imaging abnormalities. The longer term clinical outcomes of COVID‐19 vaccine‐associated myocarditis should be investigated, including symptom persistence, cardiac investigation abnormalities over time, and long term complications.
Box 1 – Characteristics of confirmed cases of COVID‐19 vaccine‐associated myocarditis in Victoria, 22 February 2021 – 30 September 2022, overall and by sex and broad age group
|
|
|
Sex |
Age group |
||||||||||||
|
All cases |
Male |
Female |
24 years or younger |
Over 24 years |
|||||||||||
|
|
|||||||||||||||
|
Number of cases |
206 |
155 [75%] |
51 [25%] |
129 [63%] |
77 [37%] |
||||||||||
|
Age (years), median (IQR) |
21 (16–32) |
19 (16–31) |
24 (21–35) |
— |
— |
||||||||||
|
Dose number |
|
|
|
|
|
||||||||||
|
First |
46 (22%) |
34 (22%) |
12 (24%) |
25 (19%) |
21 (27%) |
||||||||||
|
Second |
138 (67%) |
107 (69%) |
31 (61%) |
94 (73%) |
44 (57%) |
||||||||||
|
Third |
22 (11%) |
14 (9%) |
8 (16%) |
10 (8%) |
12 (16%) |
||||||||||
|
Vaccine |
|
|
|
|
|
||||||||||
|
AZD122 |
5 (2%) |
4 (3%) |
1 (2%) |
2 (2%) |
3 (4%) |
||||||||||
|
BNT162b2 |
171 (83%) |
131 (84%) |
40 (78%) |
109 (84%) |
62 (80%) |
||||||||||
|
mRNA‐1273 |
30 (15%) |
20 (13%) |
10 (20%) |
18 (14%) |
12 (16%) |
||||||||||
|
Brighton Collaboration level |
|
|
|
|
|
||||||||||
|
Level 1 (definitive) |
68 (33%) |
60 (39%) |
8 (16%) |
51 (40%) |
17 (22%) |
||||||||||
|
Level 2 (probable) |
138 (67%) |
95 (61%) |
43 (84%) |
78 (60%) |
60 (78%) |
||||||||||
|
Hospital admissions |
129 (63%) |
101 (65%) |
28 (55%) |
83 (64%) |
46 (60%) |
||||||||||
|
Length of hospital stay (days), median (IQR) |
2 (1–3) |
2 (1–3) |
2 (1–2) |
2 (1–3) |
2 (1–3) |
||||||||||
|
Intensive care unit admissions |
5 (2%) |
1 (1%) |
4 (8%) |
3 (2%) |
2 (3%) |
||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019; IQR = interquartile range. |
|||||||||||||||
Box 2 – Symptoms at presentation by people with confirmed COVID‐19 vaccine‐associated myocarditis, Victoria, 22 February 2021 – 30 September 2022, by sex and broad age group*

COVID‐19 = coronavirus disease 2019.* The data underlying this graph are including in the Supporting Information, table 1.
Box 3 – Influence of sex and age group on time to symptom onset, presentation symptoms, and cardiac investigations for people with confirmed COVID‐19 vaccine‐associated myocarditis, Victoria, 22 February 2021 – 30 September 2022: regression analyses including the covariates age group and sex
|
Characteristic |
Adjusted odds ratio or coefficient* (95% CI) |
||||||||||||||
|
|
|||||||||||||||
|
Time to symptom onset |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.31 (0.41–2.21) |
||||||||||||||
|
Sex (male v female patients) |
0.24 (–0.77 to 1.25) |
||||||||||||||
|
Troponin level increase |
–0.0002 (–0.0007 to 0.0002) |
||||||||||||||
|
Troponin level increase (≤ 5‐fold v > 5‐fold) |
–0.09 (–0.11 to 0.93) |
||||||||||||||
|
Symptoms at hospital presentation † |
|
||||||||||||||
|
Chest pain |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
0.98 (0.95–1.003) |
||||||||||||||
|
Sex (male v female patients) |
1.01 (0.98–1.04) |
||||||||||||||
|
Palpitations |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.18 (1.04–1.34) |
||||||||||||||
|
Sex (male v female patients) |
0.77 (0.66–0.89) |
||||||||||||||
|
Dyspnoea |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.005 (0.87–1.15) |
||||||||||||||
|
Sex (male v female patients) |
1.002 (0.86–1.17) |
||||||||||||||
|
Diaphoresis |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.19 (1.08–1.31) |
||||||||||||||
|
Sex (male v female patients) |
1.01 (0.90–1.13) |
||||||||||||||
|
Cardiac investigations |
|
||||||||||||||
|
Troponin level increase |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
–82.8 (–368 to 203) |
||||||||||||||
|
Sex (male v female patients) |
84.9 (–234 to 404) |
||||||||||||||
|
Abnormal electrocardiogram |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.07 (0.93–1.24) |
||||||||||||||
|
Sex (male v female patients) |
0.75 (0.64–0.89) |
||||||||||||||
|
Abnormal echocardiogram |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
0.98 (0.87–1.11) |
||||||||||||||
|
Sex (male v female patients) |
0.91 (0.80–1.04) |
||||||||||||||
|
Abnormal cMRI |
|
||||||||||||||
|
Age group (> 24 years v ≤ 24 years) |
1.16 (0.96–1.39) |
||||||||||||||
|
Sex (male v female patients) |
0.87 (0.70–1.07) |
||||||||||||||
|
Abnormal echocardiography result |
1.13 (0.90–1.43) |
||||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; cMRI = cardiac magnetic resonance imaging. * Time to symptom onset and troponin level increase as abnormal cardiac investigation result: linear regression (coefficient with 95% CI); symptoms at presentation and abnormal cardiac investigation results other than troponin level: logistic regression (adjusted odds ratio with 95% CIs). Bold: statistically significant (linear regression: 95% CI does not include 1; logistic regression: 95% CI does not include 0). Regression including interaction terms are included in the Supporting Information, table 2. † Emergency department, ward, or intensive care unit. |
|||||||||||||||
Box 4 – Troponin level increases in people with confirmed COVID‐19 vaccine‐associated myocarditis, Victoria, 22 February 2021 – 30 September 2022, by sex, broad age group, and cardiac magnetic resonance imaging result*
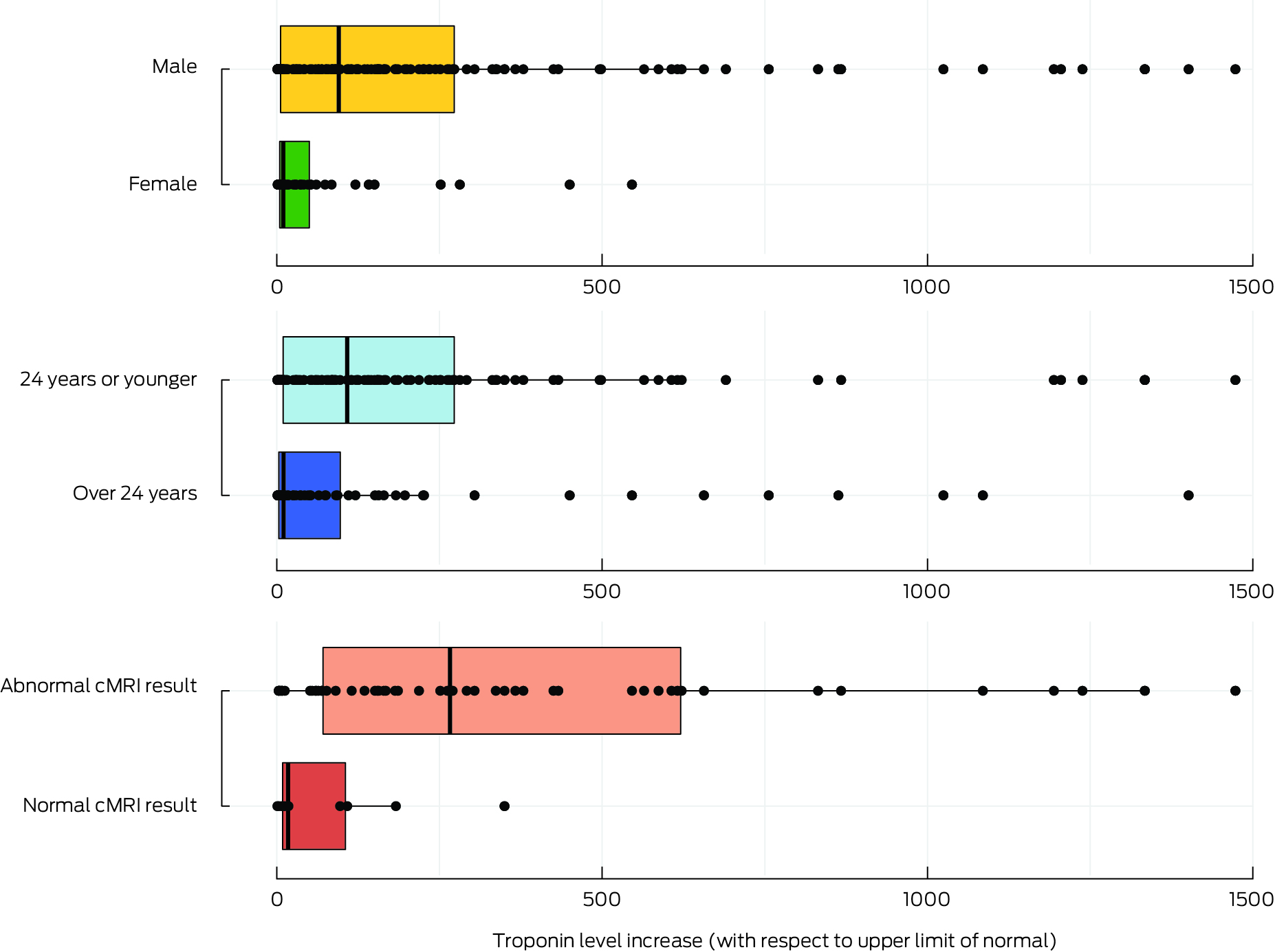
* Boxes indicate the median increase in troponin level (with interquartile range) by category, age groups and cardiac magnetic resonance imaging (cMRI) results; statistical outliers (beyond the cut‐off value defined by Tukey's rule) are not shown.
Box 5 – Abnormal cardiac investigation results in people with confirmed COVID‐19 vaccine‐associated myocarditis, Victoria, 22 February 2021 – 30 September 2022, by result type*
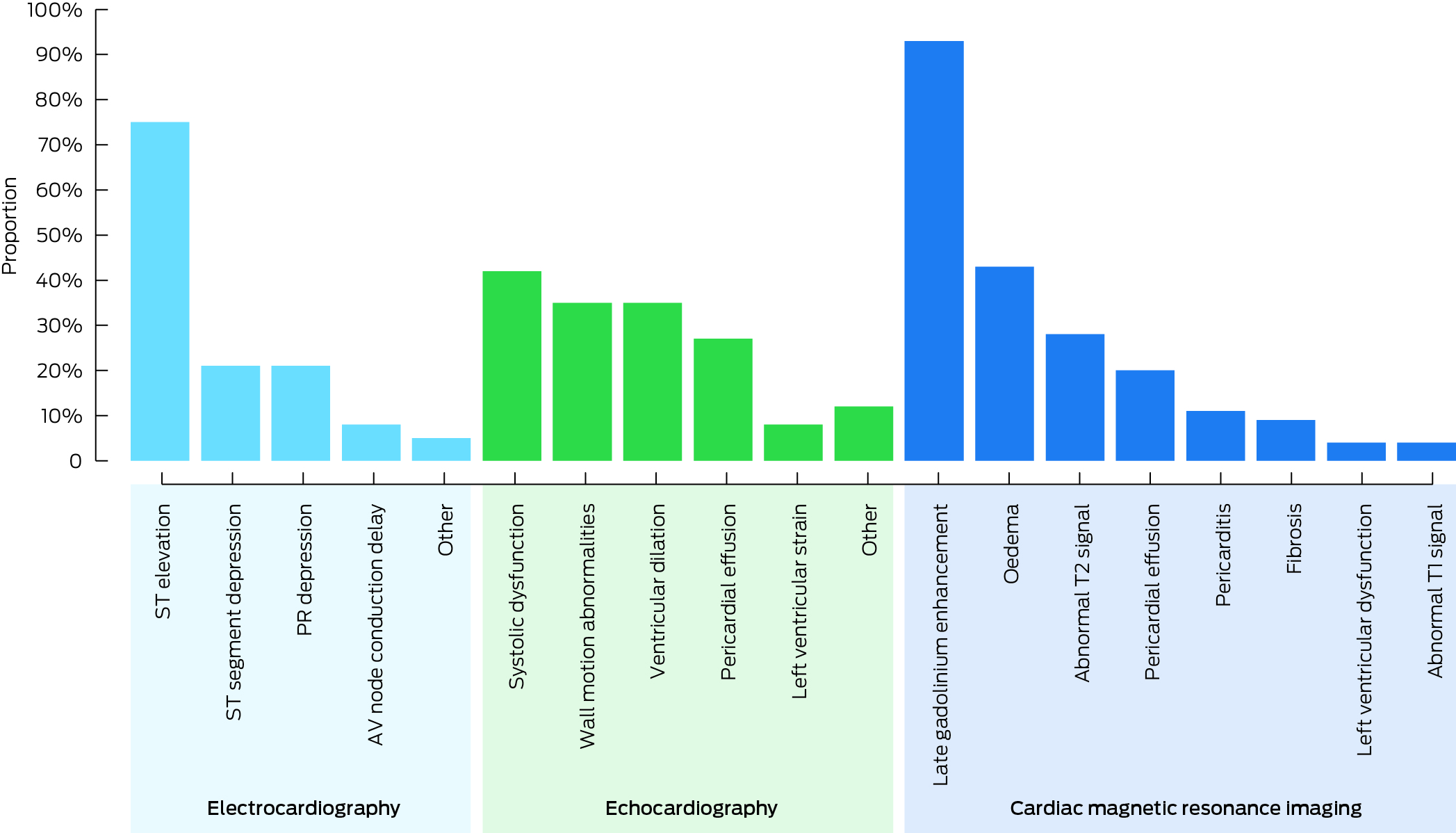
* Reported as proportions of abnormal test results reviewed. The data underlying this graph are including in the Supporting Information, table 3.
Received 25 May 2023, accepted 8 April 2024
- Julia Smith1
- Silja Schrader2
- Hannah Morgan2
- Priya Shenton1
- Annette Alafaci3
- Nicholas Cox4,5
- Andrew J Taylor6
- James Hare7
- Bryn Jones1
- Nigel W Crawford1,2
- Jim P Buttery2
- Hazel J Clothier2,8
- Daryl R Cheng1
- 1 Royal Children's Hospital Melbourne, Melbourne, VIC
- 2 Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC), Murdoch Children's Research Institute, Melbourne, VIC
- 3 Murdoch Children's Research Institute, Melbourne, VIC
- 4 Footscray Hospital, Melbourne, VIC
- 5 Melbourne Medical School, the University of Melbourne, Melbourne, VIC
- 6 Alfred Health, Melbourne, VIC
- 7 Alfred Hospital, Melbourne, VIC
- 8 The University of Melbourne, Melbourne, VIC
Data Sharing:
The ethics approval for this study does not allow sharing of individual‐level data.
We thank the Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC) nursing staff for their assistance.
No relevant disclosures.
- 1. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid‐19 in Israel. N Engl J Med 2021; 385: 2140‐2149.
- 2. Karlstad Ø, Hovi P, Husby A, et al. SARS‐CoV‐2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol 2022; 7: 600‐612.
- 3. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA‐based COVID‐19 vaccination in the US from December 2020 to August 2021. JAMA 2022; 327: 331‐340.
- 4. Shimabukuro TT. COVID‐19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP) meeting, 23 June 2021. https://stacks.cdc.gov/view/cdc/108329 (viewed July 2022).
- 5. Australian National Audit Office. Australia's COVID‐19 vaccine rollout: performance audit report. 17 Aug 2022. https://www.anao.gov.au/work/performance‐audit/australia‐covid‐19‐vaccine‐rollout (viewed Aug 2022).
- 6. Clothier HJ, Crawford NW, Kempe A, Buttery JP. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep 2011; 35: 294‐298.
- 7. Sexson Tejtel SK, Munoz FM, Al‐Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022; 40: 1499‐1511.
- 8. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377‐381.
- 9. Harris PA, Taylor R, Minor B, et al; REDCap Consortium. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019; 95: 103208.
- 10. Australian Department of Health. Australia's COVID‐19 vaccine program, COVID‐19 vaccine roll‐out. 29 Sept 2022. https://www.health.gov.au/sites/default/files/documents/2022/09/covid‐19‐vaccine‐rollout‐update‐29‐september‐2022.pdf (viewed Oct 2022).
- 11. Clothier HJ, Morgan HJ, Voss L, et al. Towards personalised medicine in vaccine safety: using pharmacoepidemiology of COVID‐19 vaccine associated myocarditis and pericarditis to inform individual risk [abstract: 22nd ISoP annual meeting, Bali, Indonesia, 6–9 November 2023]. Drug Saf 46: 1184.
- 12. Australian Department of Health. COVID‐19 vaccine safety report: 23‐09‐2022. 23 Sept 2022. https://www.tga.gov.au/news/covid‐19‐vaccine‐safety‐reports/covid‐19‐vaccine‐safety‐report‐23‐09‐2022 (viewed Nov 2022).
- 13. Cheng DR, Clothier HJ, Morgan HJ, et al; SAEFVIC and VicSIS investigators. Myocarditis and myopericarditis cases following COVID‐19 mRNA vaccines administered to 12–17‐year olds in Victoria, Australia. BMJ Paediatrics Open 2022; 6: e001472.
- 14. Nygaard U, Holm M, Bohnstedt C, et al. Population‐based incidence of myopericarditis after COVID‐19 vaccination in Danish adolescents. Pediatr Infect Dis J 2022; 41: e25‐e28.
- 15. Khorolsky CK, Shi J, Chkhikvadze T. Trends in hospitalization costs, length of stay and complications among patients with acute myocarditis: a 10‐year United States perspective [poster abstract: ACC.19, New Orleans, 16–18 March 2019]. J Am Coll Cardiol 2019; 73: 935.
- 16. Klugman D, Berger JT, Sable CA, et al. Pediatric patients hospitalized with myocarditis: a multi‐institutional analysis. Pediatr Cardiol 2010; 31: 222‐228.
- 17. Ozierański K, Tymińska A, Skwarek A, et al. Sex differences in incidence, clinical characteristics and outcomes in children and young adults hospitalized for clinically suspected myocarditis in the last ten years: data from the MYO‐PL nationwide database. J Clin Med 2021; 10: 5502.
- 18. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID‐19 mRNA vaccines. Circulation 2021; 144: 471‐484.
- 19. Kobayashi D, Aggarwal S, Kheiwa A, Shah N. Myopericarditis in children: elevated troponin I level does not predict outcome. Pediatr Cardiol 2012; 33: 1040‐1045.
- 20. Lauer B, Niederau C, Kühl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997; 30: 1354‐1359.
- 21. Pompa AG, Beerman LB, Feingold B, Arora G. Electrocardiogram changes in pediatric patients with myocarditis. Am J Emerg Med 2022; 59: 49‐53.
- 22. Mendes LA, Dec GW, Picard MH, et al. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J 1994; 128: 301‐307.
- 23. Blissett S, Chocron Y, Kovacina B, Afilalo J. Diagnostic and prognostic value of cardiac magnetic resonance in acute myocarditis: a systematic review and meta‐analysis. Int J Cardiovasc Imaging 2019; 35: 2221‐2229.
- 24. Shenton P, Schrader S, Smith J, et al; SAEFVIC VicSIS investigators. Long term follow up and outcomes of COVID‐19 vaccine associated myocarditis in Victoria, Australia: a clinical surveillance study. Vaccine 2023; 42: 522‐528.





Abstract
Objectives: To describe myocarditis as an adverse event after coronavirus disease 2019 (COVID‐19) vaccination, including a detailed description of clinical phenotypes and diagnostic test results and differences by age, sex, and degree of troponin level elevation.
Study design: Retrospective cross‐sectional study.
Setting, participants: Cases of suspected myocarditis following the administration of a COVID‐19 vaccine in Victoria during 22 February 2021 – 30 September 2022 reported to Surveillance of Adverse Events Following Vaccination In the Community (SAEFVIC), with symptom onset within 14 days of vaccination, and deemed to be confirmed myocarditis according to the Brighton Collaboration Criteria.
Main outcome measures: Demographic (sex, broad age group), vaccine, and clinical presentation characteristics; cardiac investigation results (troponin levels, electrocardiography, echocardiography, cardiac magnetic resonance imaging [cMRI]).
Results: Of 454 SAEFVIC reports of suspected COVID‐19 vaccine‐associated myocarditis, 206 were deemed confirmed cases. The median age of people with confirmed myocarditis was 21 years (interquartile range [IQR], 16–32 years; range, 10–76 years); 129 were aged 24 years or younger (63%), 155 were male (75%). The median time from vaccination to symptom onset was two days (IQR, 1–4 days); 201 cases (98%) followed the administration of mRNA vaccines; five cases followed vaccination with AZD122. Forty‐six cases followed first vaccine doses (22%), 138 second doses (67%), and 22 cases third vaccine doses (11.0%). In 201 cases, people initially presented to emergency departments; 129 people were admitted to hospital (63%; median length of stay, two days; IQR, 1–3 days). Five people were admitted to intensive care. Echocardiographic abnormalities were identified in 26 of 200 patients (13%); electrocardiographic abnormalities were identified in 105 of 206 patients (51%; less frequently in female than male patients: adjusted odds ratio, 0.75; 95% confidence interval, 0.64–0.89). Troponin levels were elevated in 205 of 206 patients; the median increase was greater in male (95.3‐fold; IQR, 5.8–273‐fold) than female patients (9.9‐fold; IQR, 4.7–50‐fold). No cMRI abnormalities were found in patients for whom the troponin increase was threefold or less.
Conclusion: The clinical severity of COVID‐19 vaccine‐associated myocarditis in Victoria was generally mild. Markers of a more severe phenotype were more frequently recorded for male patients and people aged 24 years or younger. A threefold troponin increase could be used as a threshold for risk stratification of people with COVID‐19 vaccine‐associated myocarditis, especially in hospitals with limited access to cMRI facilities.