Introduction
Scabies is a cutaneous infestation caused by the ectoparasite Sarcoptes scabiei var. hominis. It is recognised by the World Health Organization (WHO) as the most common neglected tropical disease (NTD) with cutaneous manifestations.1 Scabies disproportionately affects socially disadvantaged populations, most notably in tropical settings, although sporadic outbreaks occur in high income country settings in overcrowded institutions such as residential aged care facilities.2,3 This narrative review provides an analysis of the epidemiology, clinical presentation and strategies employed to identify and control scabies.
Methods
References for this review were identified through searches of PubMed for articles published using the term “scabies”. Reference lists of identified articles were reviewed to identify additional relevant material. No language or date range restrictions were imposed.
Biology
Humans are the sole documented reservoir for the Sarcoptes scabiei var. hominis and transmission is most commonly through direct skin contact. Overcrowding therefore substantially increases the likelihood for transmission. Higher humidity may also prolong mite survival.4 Adult female mites are about 0.4 mm in length and are not visible to the naked eye (Box 1). They burrow deep into the epidermis, forming a tunnel parallel to the stratum corneum where they deposit two to four eggs per day. Larvae hatch two to four days after the eggs have been laid and adult mites develop ten to 14 days later. Female mites can live for four to six weeks on a human host. The average adult human infested with scabies has a burden of ten to 12 mites during the first three months of infestation.5
Epidemiology
A lack of specific diagnostic criteria for scabies has contributed to inconsistent population level mapping and incomplete estimates of the current global health burden of scabies. Nonetheless, a recent systematic review estimated the global prevalence of scabies to be about 200 million people, notably widespread in Asia, Africa, Oceania and Latin America.2 The annual global incidence rate has been reported as 455 million6 and no difference was noted in the proportion of individuals with scabies in the dry versus the wet season.7 There is, however, a large variation in the scabies prevalence across populations.7 Epidemiological studies have consistently demonstrated that the prevalence of scabies is highest in young children. The highest point prevalence of scabies has been in Panama (78% in children aged < two years), Fiji (44% in children aged five to nine years) and remote communities in Australia (33% in Aboriginal children).8 The highest skin burden of scabies is in infancy and the first year of life. The Healthy Skin Project in East Arnhem Land identified that, on average, children presented at least three times for review of scabies before the age of one year.9
Impact of scabies
Scabies causes both short term impacts and long term sequalae. There is evidence that scabies causes considerable stigma, discrimination and psychological impact, but further research is needed to fully understand this burden.10,11,12 Scabies also causes economic losses due to itch, sleep disturbance and absenteeism from both school and work, although there are limited data to account for this.10,11,12
Scabetic lesions and epidermal erosion from scratching can provide a portal for bacterial entry, leading to secondary bacterial infection manifested as impetigo, which in turn can lead to cellulitis and sepsis. Secondary infection with Streptococcus pyogenes can also lead to the immune‐mediated complication of acute poststreptococcal glomerulonephritis (APSGN).13,14 It is proposed that APSGN contributes to chronic kidney disease in Indigenous populations of Australia.15 Aboriginal Australians are four times more likely to die from chronic kidney disease compared with their non‐Indigenous counterparts, a figure likely to be an underestimate.16 Skin infection by S. pyogenes may potentially lead to acute rheumatic fever, which subsequently progresses to rheumatic heart disease resulting in premature mortality. Age‐standardised incidence for rheumatic fever (< 45 years) was 124 times higher, and rheumatic heart disease prevalence (< 55 years) 61 times higher, among Indigenous compared with non‐Indigenous Australians, with the burden substantially higher in northern, remote Australian regions.17 Taken together, scabies therefore represents a major risk factor that may contribute to the persistent mortality gap between Indigenous and non‐Indigenous Australians.18
Efforts at global cost estimates have limited their scope at analyses considering scabies alone (itch) without considering secondary infection with impetigo, let alone the more serious complications. It is estimated that scabies accounts for about 3.8 million disability‐adjusted life years, lending support to the recommendation that scabies should be categorised as a Category A neglected tropical disease by the World Health Organization (WHO).2,8
Clinical presentation
Presentation with a primary infestation of scabies occurs four to six weeks after initial infection. It is characterised by a generalised intractable pruritus, perceived to be more intense at night.
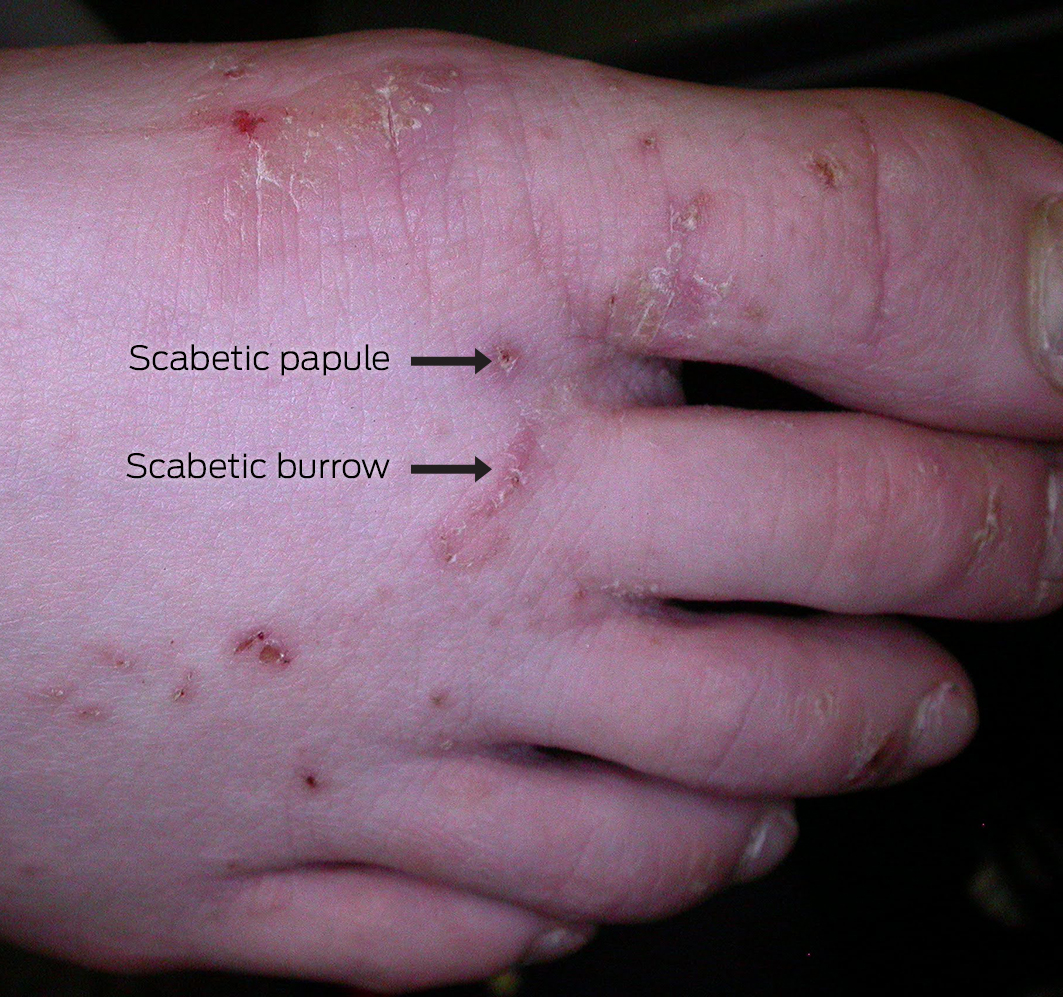
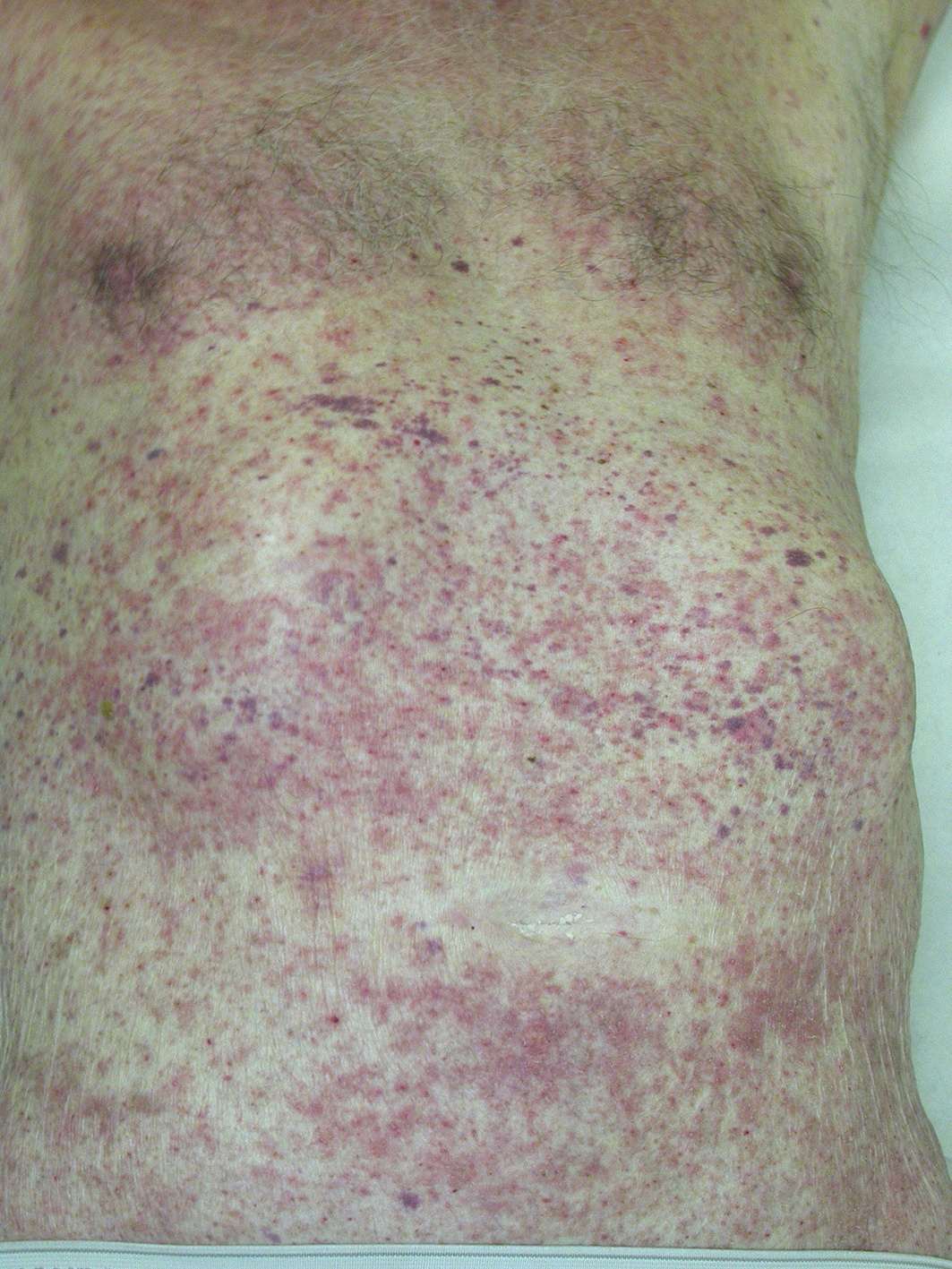
Scabies most frequently presents as papules, most commonly in interdigital web spaces, volar aspects of the wrists, sides of the hands and feet, ankles, intertriginous areas such as axillary folds or intergluteal cleft, and the genital region (Box 1).5 In infants, lesions may appear as vesicles, including on the palms of the hands and the feet, as well as on the head; this distribution is unusual in older children and adults. Nodular scabies is uncommon but can develop behind the elbows or in the genital region on the penis or scrotum.
Pruritus triggers excoriation of cutaneous manifestations and these can become eczematised due to a hypersensitivity reaction to mite components, such as saliva, eggs or faeces (Box 2). This can be further complicated by secondary infection due to a breach in the skin barrier, where cutaneous manifestations can present with erythema, pus or crusting.
Reinfestation produces an immediate hypersensitivity reaction, even with lower mite counts, suggesting the involvement of Type I and Type IV hypersensitivity responses.19 Scabies‐specific IgG and IgE levels have been observed in blood, with eosinophils, T cells, macrophages, monocytes and mast cells noted in scabies‐infested skin, driving the release of T helper 2 (Th2) and T helper 1 (Th1) immunoregulatory cytokines.19
Crusted scabies is an uncommon manifestation of scabies, occurring primarily in immunocompromised patients, for example individuals with human immunodeficiency virus infection, or older, frail patients in aged care facilities5,6 Crusted scabies is discussed further below.
Diagnosis of scabies
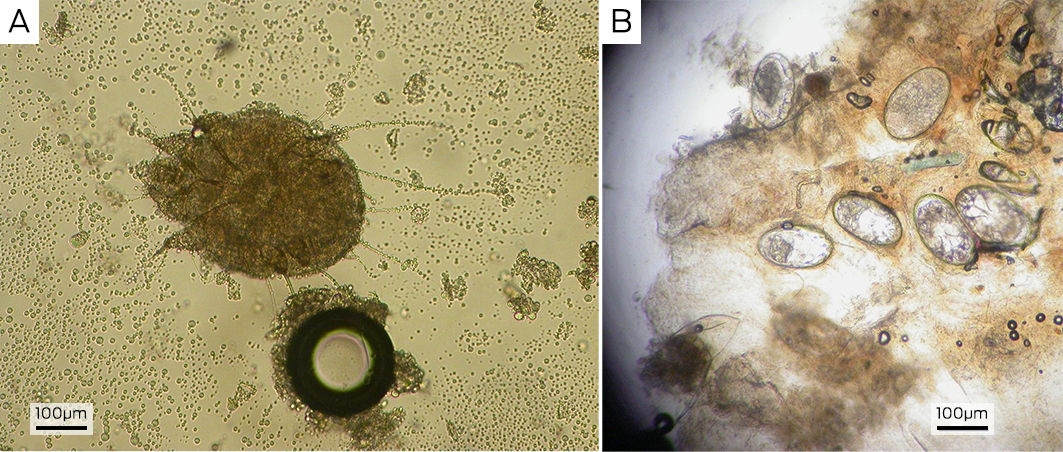
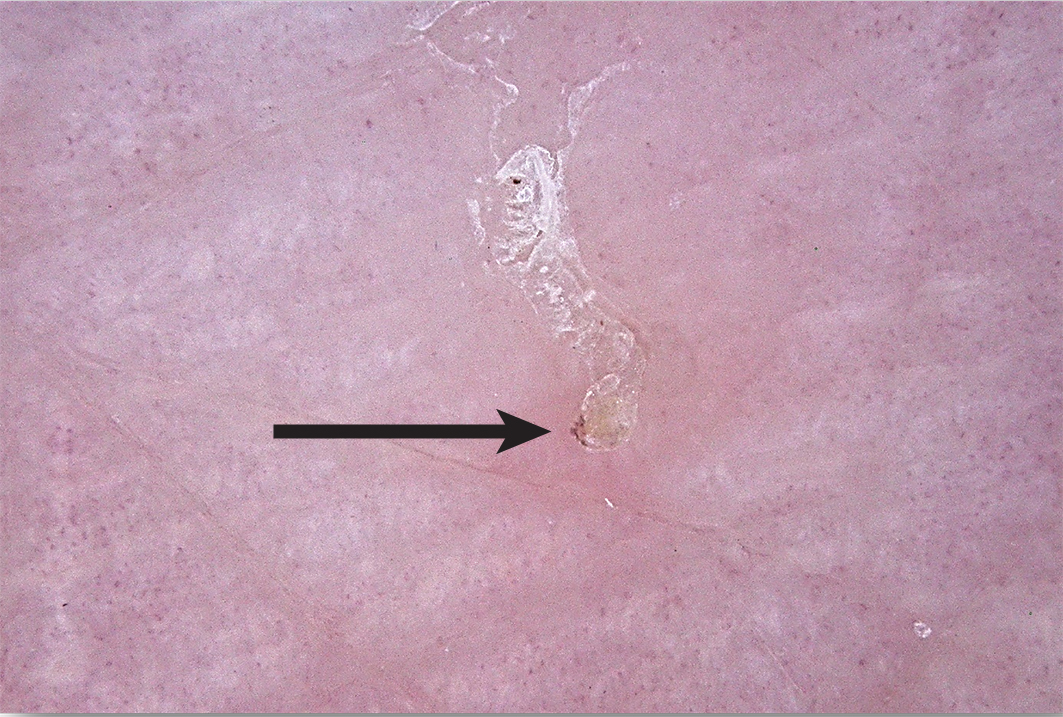
Scabies is generally a clinical diagnosis. Direct visualisation of a fresh skin scraping with 10% potassium hydroxide under light microscopy can confirm the diagnosis (Box 3, A and B); however, this is invasive and can be unreliable and impractical, especially in resource‐poor settings. The use of dermoscopy can reveal serpiginous burrows (representing the burrowing path of the adult female mite). Burrows are short, silvery‐grey, thread‐like cutaneous trails ranging about 3 to 7 mm long and 0.4 mm wide. It may also be possible to see a triangular or “delta” sign within the burrow, indicative of the mite's head (Box 4). Translucent ovoid eggs can also be seen as “mini triangular signs” under dermoscopy. Excoriation and secondary infection can alter the clinical presentation and obscure the dermoscopic presentation of scabies.6,20
In addition to dermoscopy, the “ink test” may assist in the identification of scabies burrows. This involves rubbing the suspected burrow with ink from a fountain or surgical marking pen, then removing excess ink with an alcohol wipe. The tracking of ink into the stratum corneum can indicate the presence of a burrow.6
Given the challenge of reliably diagnosing scabies, the International Alliance for the Control of Scabies consensus criteria were developed using a Delphi approach, with contribution from international experts.21 The criteria are an important advance to attempt to standardise epidemiological data and to inform evaluation of strategies for population‐level control. The current 2020 criteria incorporate three levels of diagnostic certainty. Confirmed scabies (level A) requires direct visualisation of the mite or its products. Clinical scabies (level B) and suspected scabies (level C) rely on clinical assessment of signs and symptoms, incorporating salient features on examination and history. A diagnosis of clinical or suspected scabies is made only if other differentials are considered less likely.21 Key differential diagnoses to consider include insect bites, atopic dermatitis, contact dermatitis, impetigo, dermatitis herpetiformis and secondary syphilis.13,21
General management considerations for scabies
Pioneering studies during the Second World War suggest that scabies is predominantly transmitted through direct person‐to‐person or sexual contact rather than by fomites.22 These studies agreed with findings from more recent studies of mass drug administration for scabies, where effective control of scabies was achieved without environmental control measures.23
Many guidelines continue to recommend environmental control measures, as live mites have been identified in the environment of patients, including on furniture, carpets and linen, particularly individuals with crusted scabies.24,25,26 Recommendations include hot laundering of linen, vacuuming of carpets and bagging non‐cleanable items such as pillows for 72 hours, the expected lifetime of the scabies mite without a host for survival.13 General measures to assist with itch control include avoidance of irritants, use of emollients, use of anti‐histamines and application of a medium potency topical steroid.13,27,28,29
Institutional outbreaks
In institutional outbreaks, such as in prisons or aged care facilities, involvement of a multidisciplinary team including the local public health unit, physicians, nursing staff and management is recommended to achieve infection control.30 Once an index case has been identified, this individual is advised to isolate for 24 hours until the first treatment is completed. All attending staff and visitors are recommended to use contact precautions during this period and also receive treatment to minimise spread.13 Children with scabies should be excluded from school until treatment has commenced and open sores should be covered with watertight dressings.30
Treatment
The first‐line treatment recommended for scabies is topical permethrin 5% cream, which should be applied to the whole body (from the jawline down for patients other than infants). It is generally well tolerated, applied overnight, and washed off after eight hours. A repeat application is recommended after seven days to eradicate newly hatched mites, although permethrin may be effective against all stages of the life cycle of S. scabiei. All household and asymptomatic contacts should be treated simultaneously to avoid re‐transmission13,26 (Box 5).
Second‐line topical treatment in Australia is benzyl benzoate 25%, which is applied for 24 hours. Benzyl benzoate can cause skin irritation, and should be diluted with water to 12.5% for children and infants.13 Other topical treatments to treat scabies are crotamiton lotion for two days, twice with a one week interval, 10% sulfur ointment for two or three weeks, and the insecticide lindane.26 These treatments have been explored as cheaper options for lower income settings compared with permethrin; however, a single blind randomised control trial of these more cost‐effective treatments demonstrated that topical permethrin was the more efficacious treatment when assessed after one to two weeks.31 Permethrin has an excellent safety profile and is the treatment of choice for pregnant and breastfeeding women and younger children.13,26,32
Australian guidelines recommend topical treatments as first‐line options for scabies treatment for all patients. Ivermectin is subsidised by the Pharmaceutical Benefits Scheme with streamlined authority for patients who have completed and failed sequential treatment with topical permethrin and benzyl benzoate and finished the most recent course of topical therapy at least four weeks before initiating oral therapy, or have a contraindication to topical treatment.33 Ivermectin is a macrocyclic lactone antiparasitic agent derived from fermentation products of the bacterium Streptomyces avermitilis. It is an effective agent against various parasites, including strongyloidiasis, onchocerciasis (river blindness), lymphatic filariasis and some soil‐transmitted helminths.13,34 A key advantage of ivermectin is its easy administration via oral formulation in contrast to topical agents, where patient adherence to treatment can affect efficacy. Oral ivermectin (200 μg/kg by weight) is given on Day 1 and repeated in one week. Two doses are given as ivermectin has no ovicidal activity. This is consistent with a randomised control trial that demonstrated that two doses of ivermectin were as effective as a single application of permethrin.35 The most common side effects reported with ivermectin include gastrointestinal disturbance and pruritus. Severe adverse effects reported with ivermectin include neurotoxicity resulting in confusion, ataxia, seizures and hypotension, although these are very rare. Due to theoretical safety concerns, ivermectin is not recommended for use in children aged under five years or weighing less than 15 kg, pregnant and breastfeeding women, and individuals who are frail; in these circumstances topical permethrin is recommended.36 However, a review of previous literature to date and more recent pharmacokinetic dosing modelling suggests that ivermectin is likely to be safe and well tolerated in young children, without any evidence of serious or long term adverse effects.37,38
A Cochrane review that included 22 small trials involving 2676 people, evaluated topical and oral drugs for treating scabies. The review concluded that topical permethrin was the most effective topical treatment compared with benzyl benzoate, crotamiton, lindane and sulfur. Fewer treatment failures were observed with oral ivermectin compared with topical treatments.39 An additional Cochrane review directly compared randomised controlled trials to assess the efficacy of topical permethrin against oral ivermectin for scabies, and it demonstrated that there was no difference in efficacy between the two treatments.40
Mass drug administration
In resource‐poor settings where scabies is endemic and there is a significant burden of disease, mass drug administration (MDA) may be considered as an approach to public health control.6,41 In these circumstances, guidelines support the use of oral ivermectin over topical agents, due to ease of administration to all community members regardless of their infection status.41 A study in Fiji found that MDA using ivermectin (two doses) was superior to MDA using permethrin (two doses).42 The success of MDA using two doses of ivermectin has been replicated in multiple studies.43,44 In addition, a systematic review and meta‐analysis of reports on the impact of MDA on scabies and impetigo between January 1970 and April 2021 demonstrated an overall relative reduction of scabies prevalence by 79%. MDA for scabies also led to a reduction in impetigo prevalence with a relative reduction of 66%.45
It is unclear whether a single dose of ivermectin for MDA for scabies will be equivalent to two doses as there are conflicting data. A study in a remote Aboriginal Australian community demonstrated that a single dose of ivermectin did not lead to a reduction in the prevalence of scabies.46 A cluster randomised non‐inferiority trial in the Solomon Islands evaluating one versus two doses of ivermectin‐based MDA did not demonstrate a reduction in scabies prevalence in either arm, likely due to re‐infestation in study subjects from people in surrounding villages who were not treated.47 By contrast, a similar non‐inferiority cluster randomised trial in a remote island of Fiji has shown that a single dose of ivermectin demonstrated similar excellent efficacy to that of two doses,48 therefore becoming the first study to lend support for consideration of a single dose regimen of ivermectin as a MDA strategy. Subsequently, a single group before–after study in Timor‐Leste of single‐dose ivermectin as part of triple therapy MDA for lymphatic filariasis (ivermectin, diethylcarbamazine citrate and albendazole) observed a substantial reduction in scabies.49
Target product profiles have been developed for diagnostics to support the scabies control strategy in two critical decision areas, including when to initiate MDA (community prevalence > 10%) and when to stop MDA (community prevalence < 2%).50
Crusted scabies
Crusted scabies is an uncommon but severe clinical manifestation of scabies infestation. Crusted scabies is caused by a hyperinfestation of millions of mites and has a distinctive clinical appearance with hyperkeratotic patches. Crusted scabies is associated with immunosuppression, and there is a well established association with human T‐cell lymphotropic virus 1 infection.51
The diagnosis of crusted scabies can be made clinically and confirmed with a positive skin scraping showing evidence of mites. Crusted scabies is a serious infection and requires expert management. Crusted scabies covering less than 10% of the body surface area in typical distribution with mild scaling may be managed in the community with appropriate supports. However, widespread distribution of crusted scabies or evidence of superimposed bacterial infection manifested by crusting or pustules requires hospitalisation.52 Crusted scabies is managed with repeated doses of oral ivermectin, topical scabicides and keratolytics, such as lactic acid, to reduce the scale.13 Antibiotic therapy for management of superimposed bacterial infection is often required.53
Emerging treatments
Spinosad acts on nicotinic acetylcholine receptors and γ‐aminobutyric acid receptors in arthropod muscular and nervous systems, leading to involuntary muscle contraction and paralysis.54 Spinosad topical suspension 0.9% has recently been approved by the United States Food and Drug Administration as a topical scabies treatment after two randomised controlled trials where the control arm was the solution vehicle.55 Although this treatment has shown significant promise, it has yet to be approved in Australia as a treatment for scabies. Studies of the efficacy of spinosad compared with permethrin for the treatment of scabies have not been done.
Moxidectin is a second‐generation macrocyclic lactone, related to ivermectin, and has been investigated in preclinical and early clinical studies for the treatment of scabies.56,57 Moxidectin has high lipophilicity and concentrates in skin. It has a prolonged half‐life such that a single dose of moxidectin is likely to be effective for scabies treatment.56 Moxidectin has been licensed by the US Food and Drug Administration for the treatment of Onchocerca volvulus infection (the cause of river blindness). Phase 2 and phase 3 clinical trials have demonstrated that moxidectin is significantly more efficacious than ivermectin in reducing skin microfilarial loads, with a comparable safety profile. At a dose of 8 μg/kg, moxidectin appears very safe, but further clinical development is required for scabies including dose‐finding studies and comparative efficacy studies.58,59,60,61
Afoxolaner, a veterinary drug used orally to treat fleas and ticks in dogs, has been studied in pigs experimentally infested with scabies and shows promise. In a recent preclinical study, a single dose of afoxolaner demonstrated better efficacy than two doses of ivermectin.62 Tea tree oil has also undergone preliminary investigation as a scabies treatment.63 A clinical trial to compare tea tree oil with permethrin in children with scabies has been registered in Australia but has not yet been completed (ACTRN12617000902392).
Prevention of scabies
Scabies is a significant global health concern that disproportionately affects socially disadvantaged and resource‐poor communities. Addressing the social determinants of health is ultimately the key strategy to prevent scabies transmission given its direct correlation with health inequities and outcomes. Distal factors include the intergenerational impact of displacement as a result of colonialisation and institutionalised racism, which are pervasive and deeply embedded in health care systems. These directly influence proximal risk factors such as poverty, overcrowded living conditions and low socio‐economic status that are well established as global risk factors for scabies.64 Prevention and control of scabies therefore represents a major global challenge. Community‐based approaches such as ivermectin‐based MDA for scabies can play a major role in reducing high levels of scabies.45 Education and empowerment of local communities and community‐led treatment strategies are critical to achieving sustained reductions in the burden of scabies.
Conclusion
Although momentum is building towards improved awareness, scabies remains an orphan disease with limited research to fully understand its enduring ramifications, and a narrow pipeline of new diagnostic and therapeutic approaches. Global collaboration with recognition of the upstream determinants of health, together with accurate population level mapping, and prompt diagnosis and treatment are essential to minimise the disease burden of scabies.
Box 3 – Photographs of skin scrapings under light microscopy depicting A) live mite; and B) ova (100 × magnification)
Box 4 – Dermatoscopic image of a scabetic burrow, arrow pointing to the “delta sign” indicative of the mite's head
Box 5 – Routine treatment recommendations for scabies in Australia
|
Medication |
Route |
Dosing and recommendations |
Advantages |
Disadvantages |
Availability |
||||||||||
|
|
|||||||||||||||
|
5% Permethrin (Lyclear) |
Topical |
|
|
|
PBS listed |
||||||||||
|
Benzyl benzoate 25% (Ascabiol, Benzemul) |
Topical |
|
|
|
PBS listed |
||||||||||
|
Ivermectin (Stromectol) |
Oral |
|
|
|
PBS subsidised if failed treatment with topical permethrin and benzyl benzoate |
||||||||||
|
|
|||||||||||||||
|
PBS = Pharmaceutical Benefits Scheme. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Laxmi Iyengar1,2
- Alvin H Chong1,3,4
- Andrew C Steer5
- 1 Skin Health Institute, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 University of Melbourne, Melbourne, VIC
- 4 St Vincent's Hospital, Melbourne, VIC
- 5 Centre for International Child Health, University of Melbourne, Melbourne, VIC
Patient consent:
The patients provided written consent for publication.
No relevant disclosures.
- 1. World Health Organization. Neglected tropical diseases [website]. Geneva: WHO, https://www.who.int/health‐topics/neglected‐tropical‐diseases#tab=tab_1 (viewed Dec 2023).
- 2. Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross‐sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17: 1247‐1254.
- 3. Cassell JA, Middleton J, Nalabanda A, et al. Scabies outbreaks in ten care homes for elderly people: a prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect Dis 2018; 18: 894‐902.
- 4. Arlian LG, Runyan RA, Achar S, Estes SA. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol 1984; 11: 210‐215.
- 5. Heukelbach J, Feldmeier H. Scabies. Lancet 2006; 367: 1767‐1774.
- 6. Engelman D, Cantey PT, Marks M, et al. The public health control of scabies: priorities for research and action. Lancet 2019; 394: 81‐92.
- 7. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J 2016; 35: 374‐378.
- 8. Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis 2015; 15: 960‐967.
- 9. Clucas DB, Carville KS, Connors C, et al. Disease burden and health‐care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bull World Health Organ 2008; 86: 275‐281.
- 10. Cox V, Fuller LC, Engelman D, et al. Estimating the global burden of scabies: what else do we need? Br J Dermatol 2021; 184: 237‐242.
- 11. Jin‐Gang A, Sheng‐Xiang X, Sheng‐Bin X, et al. Quality of life of patients with scabies. J Eur Acad Dermatol Venereol 2010; 24: 1187‐1191.
- 12. Lake SJ, Engelman D, Sokana O, et al. Health‐related quality of life impact of scabies in the Solomon Islands. Trans R Soc Trop Med Hyg 2022; 116: 148‐156.
- 13. Hardy M, Engelman D, Steer A. Scabies: a clinical update. Aust Fam Physician 2017; 46: 264‐268.
- 14. Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis 2012; 25: 145‐153.
- 15. Hoy WE, White AV, Dowling A, et al. Post‐streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int 2012; 81: 1026‐1032.
- 16. Hoy WE, Mott SA, McDonald SP. An update on chronic kidney disease in Aboriginal Australians. Clin Nephrol 2020 Supplement‐Jan; 93: 124‐128.
- 17. Katzenellenbogen JM, Bond‐Smith D, Seth RJ, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc 2020; 9: e016851.
- 18. Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander Health Performance Framework: summary report. Australian Government. 2024. https://www.indigenoushpf.gov.au/report‐overview/overview/summary‐report (viewed Dec 2023).
- 19. Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol 2010; 32: 532‐540.
- 20. Fox G. Diagnosis of scabies by dermoscopy. BMJ Case Rep 2009; 2009: bcr0620080279.
- 21. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 international alliance for the control of scabies consensus criteria for the diagnosis of scabies. Br J Dermatol 2020; 183: 808‐820.
- 22. Mellanby K. Transmission of scabies. Br Med J 1941; 2: 405‐406.
- 23. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med 2015; 373: 2305‐2313.
- 24. Centres for Disease Control and Prevention. Preventing scabies [website]. United States Government. https://www.cdc.gov/scabies/prevention/?CDC_AAref_Val=https://www.cdc.gov/parasites/scabies/prevent.html (viewed Dec 2023).
- 25. World Health Organization. Scabies [website]. Geneva: WHO, 2023. https://www.who.int/news‐room/fact‐sheets/detail/scabies (viewed Dec 2023).
- 26. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2015; 64: 1‐137.
- 27. Commens C. The treatment of scabies. Aust Prescr 2000; 23: 33‐35.
- 28. Chouela E, Abeldaño A, Pellerano G, Hernández MI. Diagnosis and treatment of scabies: a practical guide. Am J Clin Dermatol 2002; 3: 9‐18.
- 29. Dressler C, Rosumeck S, Sunderkötter C, et al. The treatment of scabies. Dtsch Arztebl Int 2016; 113: 757‐762.
- 30. The Australian Healthy Skin Consortium. National healthy skin guideline for the prevention, treatment and public health control of impetigo, scabies, crusted scabies and tinea for indigenous populations and communities in Australia (2nd edition). Telethon Kids Institute. October 2023. https://infectiousdiseases.thekids.org.au/resources/skin‐guidelines/ (viewed Dec 2023).
- 31. Mila‐Kierzenkowska C, Woźniak A, Krzyżyńska‐Malinowska E, et al. Comparative efficacy of topical pertmehrin, crotamiton and sulfur ointment in treatment of scabies. J Arthropod Borne Dis 2017; 11: 1‐9.
- 32. Briggs GC, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2011.
- 33. Pharmaceutical Benefits Scheme. Ivermectin (2868Y). Australian Government, Department of Health and Aged Care. https://www.pbs.gov.au/medicine/item/2868y (viewed Dec 2023).
- 34. Campbell WC. Lessons from the history of ivermectin and other antiparasitic agents. Annu Rev Anim Biosci 2016; 4: 1‐14.
- 35. Ranjkesh MR, Naghili B, Goldust M, Rezaee E. The efficacy of permethrin 5% vs. oral ivermectin for the treatment of scabies. Ann Parasitol 2013; 59: 189‐194.
- 36. Patel VM, Lambert WC, Schwartz RA. Safety of topical medications for scabies and lice in pregnancy. Indian J Dermatol 2016; 61: 583.
- 37. Wilkins AL, Steer AC, Cranswick N, Gwee A. Question 1: Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Arch Dis Child 2018; 103: 514‐519.
- 38. Gwee A, Duffull S, Zhu X, et al. Population pharmacokinetics of ivermectin for the treatment of scabies in Indigenous Australian children. PLoS Negl Trop Dis 2020; 14: e0008886.
- 39. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev 2007; 3: CD000320.
- 40. Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev 2018; 4: CD012994.
- 41. World Health Organization. WHO informal consultation on a framework for scabies control: World Health Organization Regional Office for the Western Pacific: Manila, Philippines, 19–21 February 2019: meeting report. Geneva: WHO, 2020. https://www.who.int/publications/i/item/9789240008069 (viewed Dec 2023).
- 42. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med 2015; 373: 2305‐2313.
- 43. Romani L, Marks M, Sokana O, et al. Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single‐arm community intervention trial. Lancet Infect Dis 2019; 19: 510‐518.
- 44. Middleton J. Can ivermectin mass drug administrations to control scabies also reduce skin and soft tissue infections? Hospitalizations and primary care presentations lower after a large‐scale trial in Fiji. Lancet Reg Health West Pac 2022; 22: 100454.
- 45. Lake SJ, Kaldor JM, Hardy M, et al. Mass drug administration for the control of scabies: a systematic review and meta‐analysis. Clin Infect Dis 2022; 75: 959‐967.
- 46. Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian Aboriginal community. PLoS Negl Trop Dis 2015; 9: e0004151.
- 47. Lake SJ, Engelman D, Zinihite J, et al. One versus two doses of ivermectin‐based mass drug administration for the control of scabies: a cluster randomised non‐inferiority trial. PLoS Negl Trop Dis 2023; 17: e0011207.
- 48. Hardy M, Samuela J, Kama M, et al. Community control strategies for scabies: a cluster randomised noninferiority trial. PLoS Med 2021; 18: e1003849.
- 49. Le B, Monteiro MA, Amaral S, et al. The impact of ivermectin, diethylcarbamazine citrate, and albendazole mass drug administration on the prevalence of scabies and soil‐transmitted helminths in school‐aged children in three municipalities in Timor‐Leste: a before–after assessment. Lancet Glob Health 2023; 11: e924‐932.
- 50. Marks M, McVernon J, McCarthy JS, et al. Diagnostics to support the control of scabies‐development of two target product profiles. PLoS Negl Trop Dis 2022; 16: e0010556.
- 51. Mollison LC, Lo ST, Marking G. HTLV‐I and scabies in Australian aborigines. Lancet 1993; 341: 1281‐1282.
- 52. Davis JS, McGloughlin S, Tong SY, et al. A novel clinical grading scale to guide the management of crusted scabies. PLoS Negl Trop Dis 2013; 7: e2387.
- 53. Bowen AC, Tong SY, Chatfield MD, Carapetis JR. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis 2014; 14: 727.
- 54. Fernando DD, Fischer K. Spinosad topical suspension (0.9%): a new topical treatment for scabies. Expert Rev Anti Infect Ther 2022; 20: 1149‐1154.
- 55. Seiler JC, Keech RC, Aker JL, et al. Spinosad at 0.9% in the treatment of scabies: efficacy results from 2 multicenter, randomized, double‐blind, vehicle‐controlled studies. J Am Acad Dermatol 2022; 86: 97‐103.
- 56. Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis 2016; 10: e0004389.
- 57. Bernigaud C, Fang F, Fischer K, et al. Preclinical study of single‐dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two‐dose ivermectin in a porcine model. PLoS Negl Trop Dis 2016; 10: e0005030.
- 58. Awadzi K, Opoku NO, Attah SK, et al. A randomized, single‐ascending‐dose, ivermectin‐controlled, double‐blind study of moxidectin in Onchocerca volvulus infection. PLoS Negl Trop Dis 2014; 8: e2953.
- 59. Opoku NO, Bakajika DK, Kanza EM, et al. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: a randomised, controlled, double‐blind phase 3 trial. Lancet 2018; 392: 1207‐1216.
- 60. Bakajika D, Kanza EM, Opoku NO, et al. Effect of a single dose of 8 mg moxidectin or 150 μg/kg ivermectin on O. volvulus skin microfilariae in a randomized trial: differences between areas in the Democratic Republic of the Congo, Liberia and Ghana and impact of intensity of infection. PLoS Negl Trop Dis 2022; 16: e0010079.
- 61. Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis 2016; 10: e0004389.
- 62. Bernigaud C, Fang F, Fischer K, et al. Efficacy and pharmacokinetics evaluation of a single oral dose of afoxolaner against Sarcoptes scabiei in the porcine scabies model for human infestation. Antimicrob Agents Chemother 2018; 62: e02334‐17.
- 63. Thomas J, Carson CF, Peterson GM, et al. Therapeutic potential of tea tree oil for scabies. Am J Trop Med Hyg 2016; 94: 258.
- 64. Gramp P, Gramp D. Scabies in remote Aboriginal and Torres Strait Islander populations in Australia: a narrative review. PLoS Negl Trop Dis 2021; 15: e0009751.





Summary