In Australia, as overseas, the number of transgender and gender‐diverse (trans) people seeking hormone therapy for gender affirmation is increasing.1 By aligning a person's physical characteristics with their gender identity, such therapy improves psychological wellbeing, reducing gender dysphoria and depression and improving quality of life.2,3 It is unknown how many trans people use testosterone therapy for gender affirmation in Australia.
The costs of medications for most medical conditions are subsidised for Australians by the federal government Pharmaceutical Benefits Scheme (PBS). Some medications, including testosterone, require PBS Authorities approval (“authority prescription”). As the PBS does not list gender affirmation as an indication for prescribing testosterone, clinicians use the authority indication “androgen deficiency due to an established pituitary or testicular disorder”, regardless of gender identity markers, in accordance with national guidelines.4
Our aim was to estimate the number of trans people who received PBS‐subsidised testosterone for gender affirmation in Australia during 1 July 2021 – 30 June 2022. Our request for PBS dispensing data was approved by the Services Australia External Request Evaluation Committee (RMS2527). The number of people supplied testosterone (item codes 10378F, 08830R, 08619P, 11740X, 10380H, 02115H, 10205D) by age group at 30 June 2022 was provided in aggregate, deidentified form. The testosterone formulations prescribed for trans people to increase testosterone concentrations to the male reference range (10–30 nmol/L) are the same as those used to treat men with hypogonadism.4 The proportion of people to whom testosterone was dispensed for whom a current or past female gender marker was recorded was determined by Services Australia; we interpreted a current or past female gender marker as a surrogate marker for a trans man.
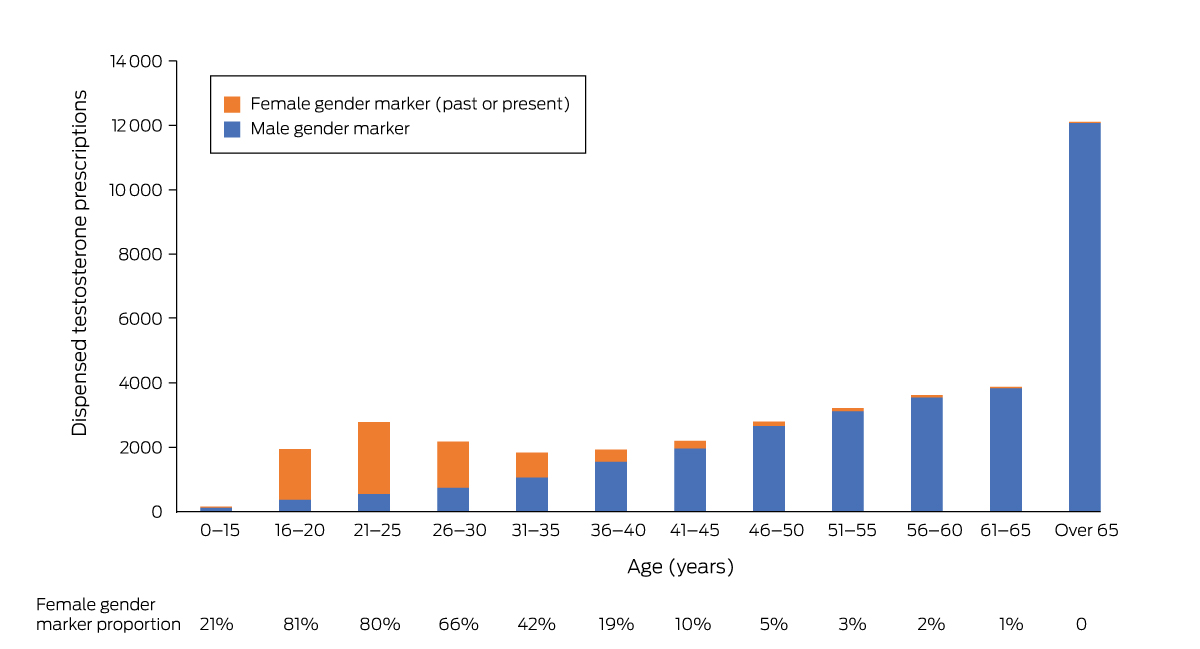
Of the 38 633 people dispensed PBS‐subsidised testosterone during 1 July 2021 – 30 June 2022, current or past female gender markers were recorded for 6998 (18%). The proportion differed markedly by age group: 6394 of 10 805 people aged 40 years or younger had current or past female gender markers (59%), including 3795 of 4726 people aged 16–25 years (80%), but only 33 of 12 114 people over 65 years of age (0.3%) (Box).
We suspect that trans people probably comprised a substantial proportion of the Australians dispensed PBS‐subsidised testosterone during 2021–22, particularly among those under 40 years of age. As there are no specific PBS items for gender affirmation treatment, people seeking medical gender affirmation may remain untreated or pay for it themselves, potentially limiting access. A specific PBS authority indication for “gender affirmation” is needed, ideally without requiring consultation with a specialist, to monitor prescribing activity for this purpose.
As limitations to our analysis we note that we could not ascertain continuity of prescriptions or off‐label prescribing. The dataset may also have included people with differences in sexual development, or women with hypopituitarism and hypoactive sexual desire disorder (androgen deficiency); however, an unsubsidised low dose testosterone formulation (AndroFeme5) would typically be used for this latter indication in Australia.
The current data do not provide a specific assessment of the number of trans people using testosterone for gender affirmation. A specific PBS authority indication for “gender affirmation” would provide an accurate assessment to guide service provision and improve quality of care. [Correction added on 28 August 2024, after first online publication: Last paragraph has been changed].
Received 19 May 2023, accepted 14 August 2023
- 1. Cheung AS, Ooi O, Leemaqz S, et al. Sociodemographic and clinical characteristics of transgender adults in Australia. Transgend Health 2018; 3: 229‐238.
- 2. Nolan BJ, Zwickl S, Locke P, et al. Early access to testosterone therapy reduces gender dysphoria, depression, and suicidality in transgender and gender diverse adults seeking masculinization: an open‐label randomized controlled trial. JAMA Netw Open 2023; 6: e2331919.
- 3. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc 2021; 5: bvab011.
- 4. Cheung AS, Wynne K, Erasmus J, et al. Position statement on the hormonal management of adult transgender and gender diverse individuals. Med J Aust 2019; 211: 127‐133. https://www.mja.com.au/journal/2019/211/3/position‐statement‐hormonal‐management‐adult‐transgender‐and‐gender‐diverse
- 5. MIMS Australia. AndroFeme 1: consumer medicine information. June 2019. https://www.nps.org.au/assets/medicines/47d5a936‐d106‐46a4‐9278‐a53300ff76e5‐reduced.pdf (viewed Nov 2023).






Open access:
Open access publishing facilitated by the University of Melbourne, as part of the Wiley – the University of Melbourne agreement via the Council of Australian University Librarians.
Brendan J Nolan holds a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship (2003939) and a Royal Australasian College of Physicians Endocrine Society of Australia Research Establishment Fellowship. Ada Cheung is supported by an NHMRC Investigator Grant (2008956). The conclusions reported in this article are those of the authors but not necessarily those of Services Australia.
No relevant disclosures.