The Australian health system is recognised as one of the best globally. However, the burden of chronic disease, including cardiovascular disease (CVD), remains high and the associated health care sector spend in Australia is rapidly expanding. In 2022–2023, Commonwealth expenditure was estimated at $132 billion, representing 16.8% of the total budget.1 Over $14 billion is spent on the direct health costs of CVD per annum.2 Developing new models to harness immense research resources available to tackle our nation's key health challenges has the potential to accelerate implementation and drive new preventive and therapeutic strategies and foster a vibrant medical technology ecosystem, thereby, positively affecting patient and economic outcomes.
Until now, there has been no mechanism that allows for a fully integrated national conversation on CVD and stroke between the health system, clinicians, researchers, industry partners, state and federal governments, and data and health economics experts. The establishment of the Cardiovascular Health Leadership Research Forum (CV HLRF) in 20223 provides new opportunities relevant to the broad range of these health care stakeholders, connecting our health leaders from all jurisdictions to our world class researchers.
The CV HLRF was designed and is hosted by the Australian Cardiovascular Alliance (ACvA) — the nation's peak body for CVD researchers. The ACvA has established a coordinated and solution‐focused model across its Flagships and Clinical Themes initiatives (Box). It is supported by senior leadership engagement from all Commonwealth, state and territory health jurisdictions, and a cash contribution from a number of jurisdictions. The total commitment to date is about $1.5 million to 2025–2026. This funding is supplemented by philanthropy and membership fees, as well as industry funding towards specific initiatives. It has engaged all relevant peak bodies across Australia, including the National Heart Foundation of Australia (NHFA), the Stroke Foundation, the National Cardiac Registry, the Australian Institute of Health and Welfare, and the Australian Commission on Safety and Quality in Health Care, who are committed to achieving unprecedented levels of collaboration towards the shared goal of optimal cardiovascular health and stroke care. It has also been endorsed by the national Health Chief Executives Forum.
Key elements of the CV HLRF include:
- endorsement by National Health Chief Executive Forum as a valuable model for connecting health, clinical and research leaders to contemporary cardiovascular health data to identify evidence‐based research and implementation priorities;
- commitment of health leaders from all Commonwealth, state and territory health jurisdictions to at least biannual forum meetings;
- collaborative approach to identifying research and implementation priorities that are deeply relevant to health system needs for CVD;
- connection to national cardiovascular research workforce through the ACvA for whole‐of‐pipeline solutions (eg, fundamental mechanisms, new diagnostic and therapeutic tools, bioengineering, clinical trials, and implementation and policy);
- building of partnerships with health services and industry and moving towards co‐commissioned approaches to prioritised areas;
- commitment to evaluating the clinical and economic impact of interventions prospectively; and
- support for an evolving national data dashboard of standardised cardiovascular outcomes and clinical quality indicators to identify major gaps and inequities.
Harmonised use of data to identify and address health challenges
There is already a wealth of information available for clinical quality improvement, but these data are often fragmented, lack robust metadata and are unable to be accessed in a systematic and timely manner. Visualisation of simplified and standardised outcomes and quality indicators will optimise its utility by health leaders, working with researchers and consumers to prioritise and solve health challenges. Disaggregated analysis of such a resource will allow for identification of inequities. This is particularly relevant for improving outcomes for Aboriginal and Torres Strait Islander Peoples, people from culturally and linguistically diverse backgrounds, and regional, rural and remote populations, as well as inequities driven by sex and gender differences and socio‐economic status. Such data can guide tailored approaches towards established clinical pathways relevant to groups with the greatest burden or inequity of health outcomes. In addition, standardised outcomes and quality indicators can also inform longer term solutions, including identifying where fundamental mechanistic studies are needed to unravel missing biology, development of new diagnostic tools, and novel drug or device solutions.
Challenges in monitoring clinical quality indicators and outcomes
National and international bodies have led the development of evidence‐based guidelines.4,5,6,7 However, there is no systematic way of comprehensively measuring compliance to guideline recommendations or to interpreting the impacts of guideline‐based care. Harmonising Australian efforts towards consensus for standardising outcome and clinical quality indicators with international organisations will enhance our participation on the global stage towards improved heart and stroke outcomes. This includes working with societies and associations such as the American College of Cardiology, the American Heart Association8 and European Society of Cardiology,9 as well as, more recently, International Consortium for Health Outcomes Measurement (ICHOM).10
The ACvA have identified five initial patient‐focused clinical challenges for optimisation of data flow and presentation to the CV HLRF: coronary artery disease/acute coronary syndrome, stroke, heart failure, arrhythmia, and sudden cardiac death. All are at different stages regarding national consensus. Even where consensus has been achieved, it is often only in one aspect of the patient journey (eg, acute coronary syndrome indicators for coronary artery disease, but no agreed indicators for primary prevention).
The establishment of the CV HLRF is a major step towards achieving consensus for standardised indicators, enabling a national conversation that can drive the development of near real‐time data dashboards. These can be implemented across all jurisdictions to ensure all Australians are receiving the best evidence‐based care. The approach is in line with the World Heart Federation, which is advocating for countries to have an increased focus on local implementation and monitoring.11
Evolving technology makes it feasible to consider automatic population of data dashboards from electronic medical records (EMR). New national integrated datasets, such as the Person Level Integrated Data Asset and the National Health Data Hub, are already available and actively being used by governments, and moving forwards, provide potential platforms for supporting consensus on standardised indicators for CVD and stroke.
Influencing policy, practice and resource allocation
Consensus on clinical quality indicators will pass a clear message to health care providers regarding their responsibility to know and report data reflecting endorsed quality indicators, but achieving this has been challenging. A key example is seen for acute coronary syndrome, where national quality indicators have been developed by leaders in the field, with wide consultation and endorsed by the Australian Commission on Safety and Quality in Health Care.12 However, few jurisdictions can report against these other than through irregular, retrospective reports, often manually populated and funded via research budgets rather than embedded into health care and budgets. The national approach to collection of standardised clinical outcomes and quality measures for stroke care has shown evidence for how effective this approach can be.13,14
Resetting expectations regarding a health provider's responsibility to know and report clinical quality data can have a significant effect. Major efficiencies can be achieved by investing in infrastructure and skills (eg, data engineers) to facilitate routine data extraction from EMRs into functional data dashboards. Integration of quality indicators into the EMR would maximise the ease and value of extraction and allow merging of registries and EMRs nationally. Improved coordination and additional investment in data infrastructure will drive broader system efficiencies and improvements in cardiovascular health. The value of such data can be seen in the SWEDEHEART cohort,15 where 1749 data points are available for over 34 000 patients with acute coronary syndrome annually (total more than 1 million patients), with long term follow‐up. Insights from these data have led to important changes in clinical practice and over 290 publications, creating an important “bank of global knowledge”. In addition, large national biobanks, such as the UK Biobank and the China Kadoorie Biobank, have been established and linked to health data and are being used to unravel the integral relationships between genetic, environmental and socio‐economic drivers of health.16,17 This type of data infrastructure also provides opportunities for embedded clinical trials.
Maximising the value and impact of medical research
The continued burden of CVD and stroke highlight the urgent need for new prevention and treatment strategies. These must be more than incremental, and require strong vision, leadership, and investment. Although the national competitive funding streams have invested significantly in research, opportunities to ensure that research addresses prioritised national inequities and unmet needs and leverages other partners for funding have not been maximised.
The members of ACvA understand the importance of working as a coordinated team within a strategic framework to make the desired impacts on the country's greatest national health challenges. The CV HLRF can rigorously establish national priorities for research and implementation, of relevance to the health system and prospectively assess impact — all in real time. This will support the development of more sustainable funding models, leveraging multiple committed partners, as research clearly defines itself as providing a valuable service to health system improvements.
The main national stream of competitive research funds for over 50 years has come from the Medical Research Endowment Account (MREA) — administered by the National Health and Medical Research Council (NHMRC). Currently, about $880 000 000 are spent on the range of funding schemes, with about 10% supporting highly competitive cardiovascular and stroke research programs. Renowned for its focus on rigor and excellence, the NHMRC system relies on investigators identifying the health problem and presenting their proposed solutions — a so‐called bottom‐up approach. A substantial number of impactful discoveries and innovations have been funded by the NHMRC. However, pathways for translation and implementation are not always clear.
The Medical Research Future Fund (MRFF) is designed as a top‐down program to complement the largely investigator‐initiated (bottom‐up) research funded by the NHMRC. The $220 million MRFF Mission for Cardiovascular Health commenced in 2021, and is contributing to further advances in reducing the burden of CVD. However, this funding only accounts for less than 0.1% of the direct cost of CVD, and we therefore need to leverage additional funds and in‐kind support to maximise impact. The Cardiovascular Health Mission has a detailed road map and implementation plan that incorporated substantial national and international consultation and feedback, which can guide and inform ambitious national collaborations. It requires sector‐wide coordination and unprecedented levels of collaboration if the ambitious goals of the Cardiovascular Health Mission and the MRFF are to be achieved.
In 2023, the federal government announced $3 billion would be allocated in its second ten‐year MRFF funding cycle with the vision: “A health system fully informed by quality health and medical research”. The CV HLRF model provides an opportunity to achieve this, leveraging the deep engagement of states and other key health, community and industry partners, and providing a strategic mechanism for connecting research to the identified problems.
The states have also invested substantially in cardiovascular research. This includes $150 million over ten years by NSW Health towards cardiovascular research capacity building, and $470 million towards an integrated Heart Hospital in Victoria. Individual states are also considering the development of state‐based CV HLRF or aligned models. Supported by the NHFA, there are now state‐based Cardiovascular Research Networks across the country. These have the potential to provide a local nexus between governments and the research sector, focusing on specific jurisdictional challenges. Queensland, New South Wales and Tasmania are rolling out statewide EMR systems. In addition, clinical and fundamental research teams have worked closely with government to establish innovative strategies and new research programs relevant to prevention in hypertension and stroke, for example. The state‐based research networks are an opportunity to align local efforts to jurisdictional priorities, use existing and emerging data infrastructure for pragmatic clinical trials and evaluation of proposed interventions, and support the national CV HLRF strategy.
Coordinated partnerships and a thriving research and development sector
Although we benefit immensely from philanthropy and government research grants, we must move away from seeing medical research as a charitable endeavour, funded in a fragmented fashion. What is needed is co‐investment in a strategic pipeline at the appropriate scale to the problems to be addressed. Given the direct health care costs of CVD are $14.3 billion per year,2 real‐time measures of the health impacts and the economic returns on investment in research and innovation will be critical to informing government policies and research and implementation prioritisation for the future. With the current approach, it is estimated that for every Australian dollar invested in health and medical research, $3.20 (Deloitte Access Economics estimate18) are returned to society in terms of better health. Notably, the highest return on investment from the NHMRC‐funded health and medical research workforce is for cardiovascular research, including stroke, yielding $9.80 per dollar invested.18 This return can be significantly enhanced by the new model of coordinated leadership via the CV HLRF. The economic benefits of a data‐driven, thriving research and development sector addressing the globe's greatest health challenge are immense and quantifiable.
Philanthropy is ready to embrace initiatives that can drive impact and scale. An exemplar in this area is the Snow Medical Research Foundation, which continues to play a leadership role through its recognition of the benefits of coordination and the need to embed research into the health system.
A proven model with relevance to chronic diseases
During the coronavirus disease 2019 (COVID‐19) pandemic, the establishment of a COVID‐19 National Cabinet played a logical and impactful role in “flattening the curve”. This was underpinned by a coordinating mechanism involving the leadership of chief medical and health officers from all jurisdictions. Gathering together experts, data and evidence helped to address the major hurdle facing coordinated health approaches in Australia: the separation of primary care, acute hospital care, and public health across the states, territories and federal jurisdictions. In this regard, the National CV HLRF can provide an exemplar for all non‐communicable diseases.
Conclusion
We urgently need a model that connects governments, health service providers and our research workforce to regularly updated data on the burden of disease and essential clinical quality indicators. The National CV HLRF does just this, allowing funders to invest in, and our health system to implement, prioritised research areas. Prioritisation and implementation will require a level of agility in resourcing and change management that is not standard in the health system. It also requires the support of a new breed of clinician‐researcher leadership, with multifaceted skills and perspectives, playing a key role in evidence‐based decision making and developing a stronger culture of mentoring early and mid‐career clinician researchers.
To support this shift, standardisation of the minimum clinical data required for monitoring outcomes and the quality of cardiovascular and stroke health services is essential. Once these indicators are clearly articulated and endorsed, they should be monitored by the individual jurisdiction and used as an opportunity for each to maximise the quality of care and outcomes and ensure equity. This is a health responsibility rather than a specific research activity. The CV HLRF can help ensure the data that are generated are used by senior leadership to guide research and innovations towards the greatest needs, with measurable improvements in outcomes. Commitment to clinical translation within the health care system is critical. Demonstrating the resulting shift in the dial will provide both health and economic benefits, contributing to future decisions, and a vibrant and sustainable research and translational ecosystem.
Box – The Australian Cardiovascular Alliance (ACvA) has designed and implemented a new model to embed research into the health system, with the centrepiece, the Cardiovascular National Health Leader Research Forum, progressively informed by dashboards of standardised outcomes and clinical quality indicators, and connected to a coordinated national cardiovascular research sector
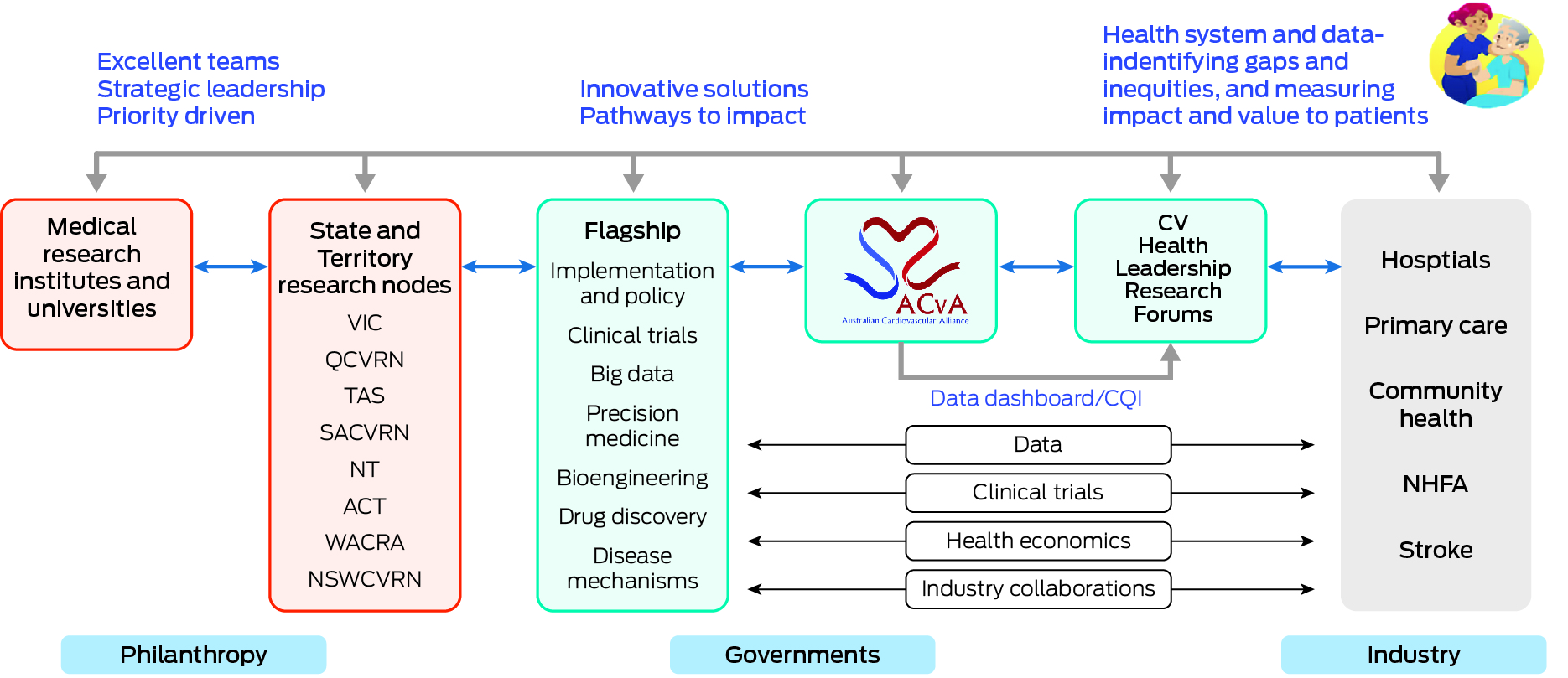
ACT = Australian Capital Territory; CQI = clinical quality improvement; CV = cardiovascular; NHFA = National Heart Foundation of Australia; NSWCVRN = New South Wales Cardiovascular Research Network; NT = Northern Territory; QCVRN = Queensland Cardiovascular Research Network; SACVRN = South Australian Cardiovascular Research Network; TAS = Tasmania; VIC = Victoria; WACRA = Western Australian Cardiovascular Research Alliance.
Provenance: Not commissioned; externally peer reviewed.
- 1. Australian Government, Department of Health. Portfolio budget statements 2022–23. Budget related paper No. 1.7: Health Portfolio. Canberra: Commonwealth of Australia, 2022. https://www.health.gov.au/sites/default/files/documents/2022/03/budget‐2022‐23‐portfolio‐budget‐statements.pdf (viewed Sept 2024).
- 2. Australian Institute of Health and Welfare. Heart, stroke and vascular disease: Australian facts [Cat. No. CVD 92; website]. Canberra: AIHW, 2023. https://www.aihw.gov.au/reports/heart‐stroke‐vascular‐diseases/hsvd‐facts (viewed June 2024).
- 3. Australian Cardiovascular Alliance. Strategic initiatives. Melbourne: ACvA, 2024. https://ozheart.org/strategic‐initiatives/health‐leadership‐research‐forum/ (viewed May 2024).
- 4. National vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. 2012. https://informme.org.au/guidelines/guideline‐for‐assessing‐and‐managing‐cardiovascular‐disease‐risk (viewed Oct 2024).
- 5. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019; 140: e596‐e646.
- 6. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Atherosclerosis 2016; 252: 207‐274.
- 7. Kanaoka K, Iwanaga Y, Tsujimoto Y, et al. Quality indicators for acute cardiovascular diseases: a scoping review. BMC Health Serv Res 2022; 22: 862.
- 8. Virani SS, Smith SC, Stone NJ, et al. Secondary prevention for atherosclerotic cardiovascular disease: comparing recent US and European guidelines on dyslipidemia. Circulation 2020; 141: 1121‐1123.
- 9. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2023; 44: 3720‐3826.
- 10. McNamara RL, Spatz ES, Kelley TA, et al. Standardized outcome measurement for patients with coronary artery disease: consensus from the International Consortium for Health Outcomes Measurement (ICHOM). J Am Heart Assoc 2015; 4: e001767.
- 11. Laranjo L, Lanas F, Sun MC, et al. World Heart Federation roadmap for secondary prevention of cardiovascular disease: 2023 update. Glob Heart 2024; 19: 8.
- 12. Australian Commission on Safety and Quality in Health Care. Acute Coronary Syndromes Clinical Care Standard (2019). Sydney: ACSQHC, 2019. https://www.safetyandquality.gov.au/publications‐and‐resources/resource‐library/acute‐coronary‐syndromes‐clinical‐care‐standard‐2019 (viewed Sept 2024).
- 13. Cadilhac DA, Andrew NE, Lannin NA, et al. Quality of acute care and long‐term quality of life and survival: the Australian Stroke Clinical Registry. Stroke 2017; 48: 1026‐1032.
- 14. Teede H, Cadilhac DA, Purvis T, et al. Learning together for better health using an evidence‐based Learning Health System framework: a case study in stroke. BMC Medicine 2024; 22: 198.
- 15. Figtree GA, Vernon ST, Hadziosmanovic N, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex‐disaggregated analysis of SWEDEHEART registry data. Lancet 2021; 397: 1085‐1094. Erratum in: Lancet 2021; 397: 1182.
- 16. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779.
- 17. Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long‐term follow‐up. Int J Epidemiol 2011; 40: 1652‐1666.
- 18. Deloite Access Economics. Australia's health and medical research workforce: Expert people providing exceptional returns. Deloitte Access Economics, 2016. https://apo.org.au/node/69841 (viewed Sept 2024).





Writing group: The complete writing group responsible for this manuscript were:
Gemma A Figtree1,2,3,4
Kerry Doyle4,5,6
David Brieger3,7
Dominique Cadilhac4,8,9,10
Kerry Chant11
Derek Chew4,10,12,13
Clara Chow3,14
Seana Gall4,13,15,16
Kim Greaves17,18,19,20
Garry Jennings3
Stefan Larson21,22,23
Jean‐Frederic Levesque11,24
Keith McNeil25
Lee Nedkoff4,26,27
Stephen J Nicholls12,13,28
Miriam Lum On29
Julie Redfern3,4,30
Christian Verdicchio3,31,32
Stephen T Vernon1,2,3
Zoe Wainer33,34
Jason Kovacic4,24,26,27,35,36,37
1 Kolling Institute of Medical Research, University of Sydney, Sydney, NSW.
2 Royal North Shore Hospital, Sydney, NSW.
3 University of Sydney, Sydney, NSW.
4 Australian Cardiovascular Alliance, Sydney, NSW.
5 Australasian Cardiac Outcomes Registry, Sydney, NSW.
6 National Institute of Complementary Medicines, Sydney, NSW.
7 Australasian Cardiac Outcomes Registry, Sydney, NSW.
8 Victorian Heart Institute, Monash University, Melbourne, VIC.
9 Monash Health, Monash University, Melbourne, VIC.
10 Australian Stroke Clinical Registry, Florey Institute of Neuroscience and Mental Health, University of Melbourne, Melbourne, VIC.
11 NSW Ministry of Health, Sydney, NSW.
12 Victorian Heart Hospital, Melbourne, VIC.
13 Monash University, Melbourne, VIC.
14 Westmead Applied Research Centre (CKC), University of Sydney, Sydney, NSW.
15 Menzies Institute for Medical Research, Hobart, TAS.
16 University of Tasmania, Hobart, TAS.
17 Queensland Health, Brisbane, QLD.
18 Sunshine Coast University, Buderim, QLD.
19 Australian National University, Canberra, ACT.
20 Sunshine Coast Hospital and Health Services, Birtinya, QLD.
21 International Consortium for Health Outcomes Measurement, Boston (MA), USA.
22 Karolinska Institute, Solna, Sweden.
23 Boston Consulting Group, Sydney, NSW.
24 UNSW Sydney, Sydney, NSW.
25 Commission on Excellence and Innovation in Health, Adelaide, SA.
26 Victor Chang Cardiac Research Institute, Sydney, NSW.
27 University of Western Australia, Perth, WA.
28 MonashHeart, Melbourne, VIC.
29 Australian Institute of Health and Welfare, Canberra, ACT.
30 George Institute of Global Health, Sydney, NSW.
31 Heart Support Australia, Canberra, ACT.
32 University of Adelaide, Adelaide, SA.
33 Victorian Department for Health, Melbourne, VIC.
34 University of Melbourne, Melbourne, VIC.
35 St Vincent's Hospital, Sydney, NSW.
36 Association of Australian Medical Research Institutes, Melbourne, VIC.
37 Cardiovascular Research Institute, Icahn School of Medicine at Mount Sinai, New York (NY), USA
We received Australian Commonwealth Government Funding through the Australian Cardiovascular Alliance non‐profit for this study.