It is estimated that 30% of infertile heterosexual couples are affected by unexplained infertility based on a diagnosis of exclusion, in the absence of abnormalities of the female and male reproductive systems after standard investigations.1 The International Committee for Monitoring Assisted Reproductive Technologies (ICMART) defined unexplained infertility as “infertility in couples with apparently normal ovarian function, fallopian tubes, uterus, cervix and pelvis and with adequate coital frequency; and apparently normal testicular function, genito‐urinary anatomy and a normal ejaculate”.2 Applying this definition requires a male and female gamete and hence the European Society of Human Reproduction and Embryology (ESHRE) and the adapted Australian guidelines have explored unexplained infertility in heterosexual couples. We acknowledge that fertility issues for those who are in a different situation are out of scope in the current guideline. We also highlight that there is no consistent understanding or standardisation of what a diagnostic workup should involve to meet this definition.
The management of unexplained infertility is likewise traditionally empirical. The efficacy, safety, costs and risks of treatment options have often not been subjected to robust evaluation and remain controversial. Existing guidelines for unexplained infertility published by the Canadian Fertility and Andrology Society in 20193 and the American Society for Reproductive Medicine in 20201 exclusively address the treatment of unexplained infertility. These two single societal evidence‐based guidelines also did not include a data integrity check or use the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework4 in formulating recommendations, and did not specifically report on primary care or consumer involvement. These gaps were addressed in the Australian evidence‐based guideline for unexplained infertility.5
Based on the lack of comprehensive evidence‐based guidelines, the ESHRE, in collaboration with the Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL) — funded by the Australian National Health and Medical Research Council (NHMRC) and led by Monash University, which provided Australian representatives throughout the ESHRE guideline development process — developed and published the ESHRE guideline for the diagnosis, assessment and treatment of unexplained infertility in 2023, focusing on both the diagnosis and the therapeutic management of couples with unexplained infertility.6,7
The CRE WHiRL sought then to address the question “what is the recommended management for couples presenting with unexplained infertility, adapted for the Australian context?”. We undertook to incorporate the assembled evidence and adapt the ESHRE guideline for the Australian context using robust international best practice, evidence‐based guideline development processes and criteria. This process included ADAPTE, the Appraisal of Guidelines for Research and Evaluation (AGREE) II, and the NHMRC and ESHRE evidence‐based guideline development methods. These met robust methodological NHMRC standards for clinical practice guidelines, and this Australian evidence‐based guideline for unexplained infertility was approved by the NHMRC.5 The guideline group engaged all relevant expertise, including general practitioners, Indigenous health care providers and consumers as well as the Fertility Society of Australia and New Zealand and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists throughout the process, across topic prioritisation, public consultation, peer review of recommendations and implementation planning and execution.
The aims of both the ESHRE and the adapted Australian guidelines are:
- to provide clinicians with evidence‐based information on the optimal diagnostic workup for infertile couples based on the examinations and procedures available to date, to correctly establish the diagnosis of unexplained infertility;
- to provide clinicians with evidence‐based information on the optimal therapeutic approach considering issues such as live birth rates, safety, patient compliance, and individualisation; and
- to adapt these recommendations for the Australian context.
Overall, the guideline aims to assist health care professionals, couples and key stakeholders in decision making about appropriate and effective management of all cases of unexplained infertility. It is still recognised that evidence‐based medical decision needs to consider individual characteristics, preferences, beliefs and values.
Methods
Detailed methods for stakeholder engagement and guideline development by ESHRE and in the ADAPTE process can be found in the guideline,5,8,9 and technical reports and administrative documents are available online at https://www.monash.edu/medicine/mchri/infertility and have been approved by the NHMRC. The Evidence Team, led by an evidence‐based specialist from ESHRE, supplemented by AM and HT (Australia), completed systematic reviews to address prioritised questions. Guideline methods aligned with best practice NHMRC requirements and AGREE II processes.4,5,6,7,8,9,10,11 Study inclusion was based on a priori population, intervention/exposure, comparison and outcome (PICO/PECO) frameworks.11 We searched online databases (including CINAHL, MEDLINE, MEDLINE In‐Process and Other Non‐Indexed Citations, PsycINFO, EMBASE and All EBM Reviews via OVID) for articles published in English language. We performed title, abstract, keyword and full text screening and the Evidence Team, the Guideline Development Group clinical leads, and evidence experts were engaged throughout the ESHRE process. This evidence review process was accepted in the Australian guideline process, except for inclusion of additional evidence related to the Australian population, setting or health system, and for exclusion of some evidence after incorporation of a research integrity process. To ensure authenticity and accuracy of evidence, the Research Integrity in Guidelines and Evidence Synthesis (RIGID) framework12 was incorporated, consistent with previously NHMRC‐approved guidelines. Here, study level integrity scores were assessed using the Trustworthiness in Randomised Controlled Trials (TRACT) tool,13 and an integrity committee for consensus on study allocations. Authors of studies with moderate and high risk of integrity issues were contacted. All scores and reasons were tabulated in the technical report available online.
In developing and interpreting the guideline in both the ESHRE and Australian ADAPTE process, evidence was evaluated alongside multidisciplinary health professional expertise and consumer perspectives in all stages from conceptualisation, development, international and Australian peer review and translation. Population, resources, health system issues, access to health care professionals, investigations and therapies were considered in Australia in the adaptation, underpinned by an agreed set of principles (Box 1), following the GRADE process.11 Three independent methodologists reviewed the Australian adaptation of the guideline during public consultation, of whom one was commissioned by the NHMRC, to optimise clarity of methods and alignment to NHMRC requirements.
In interpreting guideline recommendations, these are presented as category, terms, GRADE and quality of evidence.
Category. These include evidence‐based recommendations (EBRs) and accompanying practice points (PPs) to guide clinical implementation (Box 2), or if inadequate evidence was available, research recommendations were made.
Terms. Aligning ESHRE and NHMRC recommendations, terms used include “should”, “could”, “probably is not” or “is not”, informed by the nature of the recommendation, the GRADE framework, and evidence quality as independent descriptors reflecting the judgement of the multidisciplinary Guideline Development Group, including consumers. They refer to overall interpretation and practical implementation, balancing benefits and harms. “Should” is used where benefits of the recommendation exceed harms, and where the recommendation can be trusted to guide practice. “Could” is used where either the quality of evidence was limited or the available studies demonstrate little clear advantage of one approach over another, or the balance of benefits to harm was unclear. “Probably is not” is used where there is no clear advantage of one option over another or the option is probably not recommended. “Is not recommended” is used where the evidence against the test or intervention suggests the harms may outweigh the benefits.
GRADE. Recommendation GRADE was determined from structured, transparent consideration of the GRADE framework, including desirable effects, undesirable effects, balance of effects, overall quality of evidence, patient values and preferences, resource requirements and cost‐effectiveness, equity, acceptability and feasibility (Box 3).
Quality of evidence. The quality of evidence (Box 4) reflected the confidence in whether an estimate of the effect is adequate to support a recommendation and was largely determined by the Expert Evidence Synthesis Team, categorised according to:
- the number and design of studies addressing the outcomes;
- judgments about quality of included studies and/or synthesised evidence, risk of bias, inconsistency, indirectness, imprecision and any other considerations that may influence the quality of the evidence;
- key statistical data; and
- classification of the importance of the outcomes.
The current guideline applies the terms and definitions as described in The international glossary on infertility and fertility care, 2017.2 Specifically, the term “medically assisted reproduction” refers to reproduction brought about through various interventions, procedures, surgeries and technologies to treat different forms of fertility impairment and infertility. These include ovulation induction, ovarian stimulation, ovulation triggering, all assisted reproductive treatments, uterine transplantation and intrauterine, intracervical and intravaginal insemination with semen of partner or donor.
The guideline itself includes the clinical need for the question, the clinical question, the evidence summary, the recommendation and practice points and a summary of the justification developed by the Guideline Development Group and modified by ESHRE international and Australian peer review. The comprehensive evidence reviews, profiles and GRADE framework supporting each recommendation can also be found in the full guideline5 and supplementary technical report.9
The summary of recommendations reflects the category, terms, GRADE and quality of the evidence (Box 5). Box 6 summarises the diagnostic process and Box 7 the key recommended treatment options.
Assessment and management recommendations
Unexplained infertility is a diagnosis made where a pregnancy does not result after a period of time in which there is regular sexual intercourse, and no abnormal pathology has been discovered after thorough investigation.2 The vagueness of this definition has caused distress to affected couples and led to excessive investigation and early intervention by many health professionals. This guideline has clarified some of the concerns of patients and their health professionals in diagnosis and management based on evidence and rigorous processes to obtain quality conclusions, the best clinical options where adequate evidence is lacking, cost‐effectiveness of investigation, and treatment and patient preferences. The latter is particularly important, and the guideline group incorporated patient and community members who actively provided their opinions, relevant for the Australian context. Important conclusions are discussed below.
Age is important, as the presence of normal tests and medical history suggests waiting up to 12 months from commencement of a sexual relationship leads to many women becoming pregnant without medical assistance in that timeframe.19 This is relevant for women up to 40 years of age, although many would consider earlier intervention in the late 30s, especially when considering aspirational family size. Given the decreasing success of medically assisted reproduction in the later reproductive years, earlier intervention may be agreed upon between the health professional and the couple. Another controversial area is the definition of regular sexual intercourse around which sensitive communication is required, involving a sexual history.
The guideline is conservative in terms of investigations. Considering benefits and risks, regular menstrual cycles may be an adequate indication of ovulation. Laparoscopy and hysteroscopy are not required in the absence of a relevant history of infection, pain or miscarriage. Ultrasonographic or radiographic visualisation of the tubes and uterus is less invasive, lower risk and less expensive than surgery, while providing adequate information. A standard semen analysis incorporating the World Health Organization criteria20 (without the need for DNA fragmentation testing) and the lack of evidence for testing for autoimmunity, other hormones and metabolites are key elements of the guideline.
The guideline is also essentially conservative in terms of treatment approaches. The unexplained nature of the condition almost certainly incorporates subgroups which currently cannot be distinguished but will need different approaches in future. Many couples, especially those younger (aged 30–35 years or less), will become pregnant naturally after time, potential lifestyle adjustment, and more precisely planned intercourse. Others, especially those who are older, have been infertile for longer, or who have never been pregnant, may have a worse prognosis for spontaneous pregnancy and may require treatment earlier, especially where multiple children are desired.
The guideline emphasises the value of stimulated intrauterine insemination (IUI) as a lower cost, effective treatment in many of these couples. Several studies have shown higher live birth rates in unexplained infertility when IUI is used compared with further expectant management, particularly in younger women.21
IUI is best performed in a specialist clinic where stimulation of the cycle with oral agents such as clomiphene or letrozole can replace the older method of gonadotrophin use, and monitoring to exclude multiple follicle formation can be pursued. In many circumstances, several cycles of IUI are as effective as stimulated in vitro fertilisation (IVF)22 and this may prove more acceptable to patients and more readily applicable to regional centres lacking sophisticated embryology laboratories.21 Failure of several IUI cycles could then necessitate the consideration of the use of IVF.
Implementation of recommendations and translation of guidelines are key to clinical and policy impact. Recommendations are supported by evidence‐based, freely accessible co‐designed resources for health professionals (algorithms, webinars, toolkits) and patients (free ASKFertility mobile phone application, fact sheets and webinars). Translation is prioritised and now funded by the Medical Research Future Fund with implementation following robust frameworks including the Consolidated Framework for Implementation Research and the Learning Health System.23 We include broad engagement with relevant stakeholders and a range of tools and strategies (https://www.monash.edu/medicine/mchri/infertility). This translation will also focus on supporting the Indigenous population, with higher prevalence of unexplained infertility, and on more limited access to care in regional and rural areas supporting those in regional, rural and remote areas. Translation outputs will target policy makers, organisations (resource kits and models of care) and individuals across training, resources and tools for health professionals (undergraduate and postgraduate) and for resources for couples focused on enhancing knowledge and facilitating shared decision making. Although challenges are acknowledged, stakeholder engagement, extensive expertise and funded strategies align to other areas of women's health where the CRE WHiRL's extensive guideline reach, advanced knowledge, new models of care and advocacy have been delivered in women's health conditions.
The overall quality of evidence is relatively limited and emphasises the critical need for greater high quality research in the field. Further standardisation of the diagnostic workup is needed alongside research on efficacy, safety and costs. This is key to being able to accurately advise couples with unexplained infertility, including on whether to wait longer, access IUI or proceed directly to IVF. The science of prognosis advice for this condition is underdeveloped,24,25 and the models that do exist are based on other countries’ health systems or outmoded testing.26 This area is a major priority across stakeholders moving forward. The cost‐effectiveness of the guideline's advice also needs testing under Australian conditions, although conservative recommendations here are likely to reduce costs and burden to both consumers and the health system. Further research is also needed into patient preferences.
Australia's health model in infertility has evolved to one of competitive commercial organisations that offer higher cost, more complicated services, with a tendency towards earlier intervention, less IUI, more sophisticated testing, and treatment by IVF. The guideline emphasises the lack of evidence to support these approaches and is relatively conservative in the robustly developed recommendations. It also highlights the critical need for more research in the field. If we are to offer accessible, equitable, cost‐effective fertility services to people who desire a child, we need to align to best practice evidence, and to evaluate current practice in the light of a guideline developed in collaboration with the leading organisation in Europe and adapted to Australian current health systems and practices.
Box 1 – Agreed principles in adapting the European Society of Human Reproduction and Embryology (ESHRE) unexplained infertility guidelines for Australia
Principles underpinning the ADAPTE process from ESHRE guideline to the Australian setting including:- Access to diagnostic assessments, treatment and monitoring of unexplained infertility are adversely impacted by regionality and rurality in Australia, which represents an equity issue and needs to be considered in making recommendations and in informing policy on fertility care in Australia.14
- Australian Aboriginal and Torres Strait Islander people are disproportionately represented in regional settings, acknowledging that most do live in urban areas. They are so disproportionately affected by a range of risk factors for infertility warranting education, health care models, policy change and further research to ensure accessible, timely and equitable care.15,16
- Inadequate information or misinformation is common in infertility, with an imperative for evidence‐based care across diagnosis, treatment and monitoring, and with a need for resources, tools and education to enable informed shared decision making between patients and health care professionals.
- Cost‐effectiveness data are limited in the Australian setting on comparisons between expectant management and different fertility options, yet health professionals should be aware of, inform and enable shared decision making encompassing direct and indirect costs.17
Box 2 – Categories of guideline recommendations*
|
|
|||||||||||||||
|
Evidence‐based recommendation (EBR): evidence sufficient to inform a recommendation made by the Guideline Development Group. |
|||||||||||||||
|
Practice point (PP): evidence not sought or insufficient to make an EBR. A PP has been made by the Guideline Development Group where important issues arose from discussion of evidence‐based recommendations. |
|||||||||||||||
|
|
|||||||||||||||
|
* Aligned to the European Society of Human Reproduction and Embryology (ESHRE) guideline, we did not employ consensus recommendation terminology. |
|||||||||||||||
Box 3 – The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework recommendation strength11

Box 4 – Quality (certainty) of evidence categories, adapted from the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework

Box 5 – Recommendations

3D = three‐dimensional; BMI = body mass index; EBR = evidence‐based recommendation; GRADE = Grading of Recommendations, Assessment, Development and Evaluation; HPV = human papilloma virus; IUI = intrauterine insemination; MRI = magnetic resonance imaging; PCOS = polycystic ovary syndrome; PP = practice point; WHO = World Health Organization. * Recommendations were adapted from the European Society of Human Reproduction and Embryology (ESHRE) guideline6,7 due to the integrity check or the GRADE consideration in the Australian context. For the legend for the GRADE/quality column, please refer to Box 3 and Box 4.Source: Recommendations from the 2024 Australian Evidence‐based guideline for unexplained infertility: ADAPTE process from the ESHRE evidence‐based guideline on unexplained infertility. Monash University (https://www.monash.edu/medicine/mchri/infertility) 2024, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This content is not covered by the terms of the Creative Commons licence of this publication. For permission regarding reuse, please contact the rights holder.
Box 6 – Algorithm: recommended tests to diagnose unexplained infertility
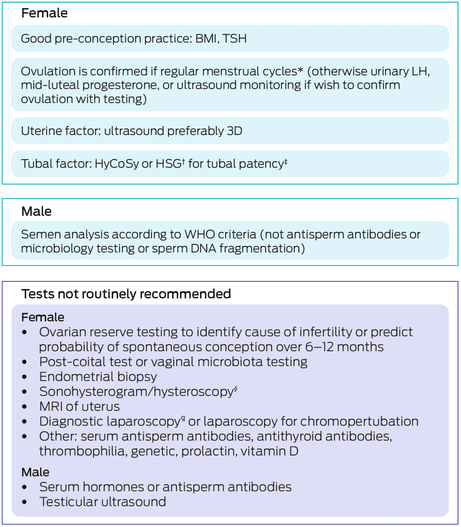
3D = three‐dimensional; BMI = body mass index; HSG = hysterosalpingography; HyCoSy = hystero‐contrast‐sonography; LH = luteinising hormone; MRI = magnetic resonance imaging; TSH = thyroid stimulating hormone; WHO = World Health Organization. * Twenty‐one to 35 days, with shortest to longest variation less than seven to nine days. † If performing an HSG, consider tubal flushing with oil‐soluble contrast medium rather than water‐soluble contrast medium. ‡ Consider Chlamydia antibody testing to differentiate between patients at low and high risk for tubal occlusion. § Consider if risk factors for intrauterine adhesions (Asherman syndrome) (ie, past intrauterine infection or surgery). ¶ Consider discussing benefits versus harms in terms of diagnosing minimal or moderate endometriosis.Source: Recommendations from the 2024 Australian Evidence‐based guideline for unexplained infertility: ADAPTE process from the ESHRE evidence‐based guideline on unexplained infertility. Monash University (https://www.monash.edu/medicine/mchri/infertility) 2024, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This content is not covered by the terms of the Creative Commons licence of this publication. For permission regarding reuse, please contact the rights holder.
Box 7 – Algorithm: recommended treatment
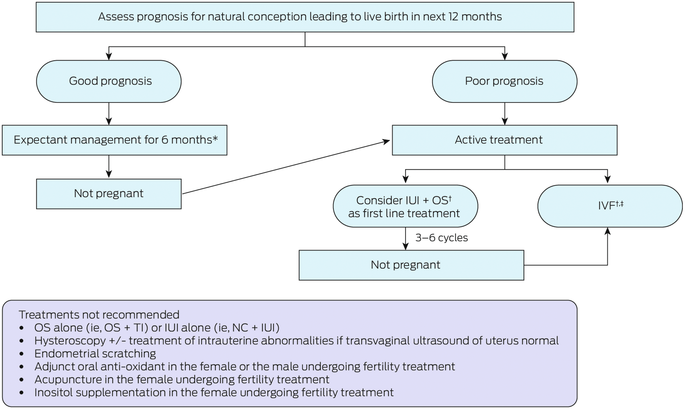
IUI = intrauterine insemination; IVF = in vitro fertilisation; NC = natural cycle; OS = ovarian stimulation; TI = timed intercourse. * Advise sexual intercourse of at least every two to three days within the fertile window. † Acknowledging that IUI + OS versus IVF for first line treatment to be individualised depending on patient characteristics (ie, age, duration of infertility, previous treatment, previous pregnancy); and benefits versus harms, patient values and preferences, cost and feasibility. ‡ Not intracytoplasmic sperm injection over that of conventional IVF.Source: Recommendations from the 2024 Australian Evidence‐based guideline for unexplained infertility: ADAPTE process from the ESHRE evidence‐based guideline on unexplained infertility. Monash University (https://www.monash.edu/medicine/mchri/infertility) 2024, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in Women's Health in Reproductive Life. This content is not covered by the terms of the Creative Commons licence of this publication. For permission regarding reuse, please contact the rights holder.
Provenance: Not commissioned; not externally peer reviewed
- Michael F Costello1,2
- Robert J Norman1,3
- Luk Rombauts1,4
- Cynthia M Farquhar5
- Lisa Bedson6
- Marlene Kong7
- Clare V Boothroyd8
- Rebecca Kerner9
- Rhonda M Garad10
- Trudy Loos11
- Madeline Flanagan5
- Ben W Mol3,4,11
- Aya Mousa1,10
- Daniela Romualdi12
- Baris Ata13
- Ernesto Bosch14
- Samuel Santos‐Ribeiro15
- Ksenija Gersak16
- Roy Homburg17
- Nathalie Le Clef18
- Mina Mincheva19
- Terhi Piltonen20
- Sara Somers21
- Sesh K Sunkara22
- Harold Verhoeve23
- Helena J Teede1,10,11
- For the Australian NHMRC Centre for Research Excellence in Reproductive Life UI Guideline Network and the ESHRE guideline group for unexplained infertility
- 1 NHMRC Centre for Research Excellence in Women's Health in Reproductive Life, Sydney, NSW
- 2 University of New South Wales, Sydney, NSW
- 3 Robinson Research Institute, University of Adelaide, Adelaide, SA
- 4 Monash University, Melbourne, VIC
- 5 University of Auckland, Auckland, New Zealand
- 6 Repromed, Adelaide, SA
- 7 Whitsundays Doctors Service, Airlie Beach, QLD
- 8 Care Fertility, Brisbane, QLD
- 9 Adelaide, SA
- 10 Monash Centre for Health Research and Implementation, Monash University, Melbourne, VIC
- 11 Monash Health, Melbourne, VIC
- 12 Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 13 Koç University Hospital, Istanbul, Turkey
- 14 IVI RMA, Valencia, Spain
- 15 IVI RMA, Lisbon, Portugal
- 16 University of Ljubljana and University Medical Centre, Ljubljana, Slovenia
- 17 Liverpool Women's Hospital, Hewitt Fertility Centre, Liverpool, United Kingdom
- 18 European Society of Human Reproduction and Embryology, Grimbergen, Belgium
- 19 London, United Kingdom
- 20 Oulu University Hospital, Medical Research Centre, University of Oulu, Oulu, Finland
- 21 Ghent University Hospital, Ghent, Belgium
- 22 King's College London, London, United Kingdom
- 23 Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
The Australian National Health and Medical Research Council (NHMRC) funded the guideline development through the Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL) (APP1171592) administered by Monash University, Australia. The NHMRC was the approver of the Guideline but had no influence on the outcome. This work builds on the work of ESHRE and sections where relevant are reproduced here with permission. Copyright of the original UI Guideline belongs to European Society of Human Reproduction and Embryology (all rights reserved). The content of the original ESHRE guidelines has been published for personal and educational use only and is adapted here with permission. No part of the ESHRE guidelines may be translated or reproduced in any form without prior written permission of the ESHRE communications manager. Monash University is subject to copyright. Monash holds the copyright for the Australian adapted UI Guideline and recommendations. No commercial use is authorised. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without written permission from ESHRE and Monash University.
Lisa Bedson is employed by Repromed Fertility Specialists. Claire Boothroyd is on the Merck‐funded male factor infertility guideline, and received speaker fees from Organon, Merck, Da Vinci, Ferring, Besins, Gideon Richter as well as private practice or professional income from Owner Care Fertility (IVF unit offering treatments). Michael Costello has received speaker honoraria from Merck/CREI. Cynthia Farquhar has been funded by Cochrane for evidence synthesis and advisory board roles; she has also been the Chair NZICA and a WHO, task force and infertility guideline member, President elect ASPIRE, and RANZCOG research and guideline lead. Robert Norman declared NHMRC funding via research grants, MRFF funding, advisory board member as Chair of the Clinical Advisory Committee at Westmead Fertility, Chair Board of HOPE Research Institute Vietnam, consulting honoraria past trainer Flinders Fertility, consultant Vinmec Hospital Vietnam and speakers fee or honoraria from several pharmaceutical companies in India. Luk Rombauts declared research grants/contracts with Monash IVF Group and was on an advisory board for Merck with private practice income from LIF Rombauts Pty Ltd. Daniela Romualdi received consulting fees from SICS Editore, UCB Pharmax, honoraria from IBSA and Novo Nordisk. Baris Ata received speakers fees from Merck, Ferring, IBSA, Organon and Abbott. Ernesto Bosch received research grants from Roche diagnostics and IBSA with consulting fees from Merck, Ferring, Gedeon Richter, Mint diagnostics and speaker's fees from Merck, Ferring, Gedeon Richter, IBSA, salary from IVI RMA Valencia, ownership by stock or partnership from IVI RMA Valencia and Mint diagnostics. Samuel Santos‐Ribeiro received research grants from MSD, Ferring, Merck, Abbott, Roche, Obseva and consulting fees from Ferring, MSD and speaker's fees from Ferring, MSD and Besins. Mina Mincheva received consulting fees from Mojo Fertility Ltd. Terhi Piltonen Research received a grant from Roche and speaker's fees from Gedeon Richter, Roche, Exeltis. Helena Teede receives competitive grant funding from government sources and holds unpaid international leadership roles with WHO and professional societies. Natalie Vujovich, Rhonda Garad, Trudy Loos, Marlene Kong, Sara Somers, Roy Homburg, Donia Scicluna, Ksenija Gersak and Nathalie Le Clef have nothing to declare.
- 1. Practice Committee of the American Society for Reproductive Medicine. Evidence‐based treatments for couples with unexplained infertility: a guideline. Fertil Steril 2020; 113: 305‐322.
- 2. Zegers‐Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril 2017; 108: 393‐406.
- 3. Buckett W, Sierra S. The management of unexplained infertility: an evidence‐based guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online 2019; 39: 633‐640.
- 4. National Health and Medical Research Council. NHMRC levels of evidence and grades for recommendations for developers of guidelines. Canberra: NHMRC, 2009. https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008‐09.pdf (viewed June 2024).
- 5. Costello MF, Teede H, Farquhar C, et al. Australian evidence‐based guideline for unexplained infertility: ADAPTE process from the ESHRE evidence‐based guideline on unexplained infertility 2024 [website]. Melbourne: Monash University, 2024. https://bridges.monash.edu/articles/online_resource/Australian_evidence‐based_guideline_for_unexplained_infertility_ADAPTE_process_from_the_ESHRE_evidence‐based_guideline_on_unexplained_infertility_2024_/26061295 (viewed June 2024).
- 6. Guideline Group on Unexplained Infertility; Romualdi D, Ata B, Bhattacharya S, et al. Evidence‐based guideline: unexplained infertility. European Society of Human Reproduction and Embryology, 2023. https://www.eshre.eu/guideline/UI (viewed Aug 2024).
- 7. Guideline Group on Unexplained Infertility; Romualdi D, Ata B, Bhattacharya S, et al. Evidence‐based guideline: unexplained infertility†. Hum Reprod 2023; 38: 1881‐1890.
- 8. ADAPTE Collaboration (2009). The ADAPTE Process: resource toolkit for guideline adaptation; version 2.0. Guideline International Network, 2010. https://g‐i‐n.net/wp‐content/uploads/2021/05/ADAPTE‐Resource‐toolkit‐V2.1‐March‐2010‐updated‐disclaimer.pdf](viewed Aug 2024).
- 9. Mousa A, Le Clef N, Costello M, et al. Technical report for the Australian Adaptation of the ESHRE Evidence‐based guideline for unexplained infertility 2024. Melbourne: Monash University, 2024. https://bridges.monash.edu/articles/report/Technical_Report_for_the_Australian_Adaptation_of_the_ESHRE_Evidence‐based_guideline_for_unexplained_infertility_2024_/26299363 (viewed June 2024).
- 10. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010; 182: E839‐E842.
- 11. Higgins J, Thomas J, Chandler J, et al; editors. Cochrane handbook for systematic reviews of interventions version 6.4 [updated Aug 2023]. Cochrane, 2023. https://training.cochrane.org/handbook/current (viewed Aug 2024).
- 12. Mousa A, Flanagan M, Tay CT, et al. Research Integrity in Guidelines and evIDence synthesis (RIGID): a framework for assessing research integrity in guideline development and evidence synthesis. Lancet eClinicalMedicine 2024; https://doi.org/10.1016/j.eclinm.2024.102717.
- 13. Mol BW, Lai S, Rahim A, et al. Checklist to assess Trustworthiness in RAndomised Controlled Trials (TRACT checklist): concept proposal and pilot. Res Integr Peer Rev 2023; 8: 6.
- 14. Lazzari E, Baffour B, Chambers GM. Residential proximity to a fertility clinic is independently associated with likelihood of women having ART and IUI treatment. Hum Reprod 2022; 37: 2662‐2671.
- 15. Gilbert E, Walker R, Simon D, et al. “We are only looking at the tip of the iceberg in infertility”: perspectives of health providers about fertility issues and management among Aboriginal and Torres Strait Islander people. BMC Health Serv Res 2021; 21: 704.
- 16. Clarke M, Whitson N, Williams C, Robson SJ. A silent burden‐prolapse, incontinence, and infertility in Australian Aboriginal and Torres Strait Islander women: a systematic search and narrative review. Int J Gynaecol Obstet 2021; 155: 268‐274.
- 17. Keller E, Botha W, Chambers GM. What features of fertility treatment do patients value? Price elasticity and willingness‐to‐pay values from a discrete choice experiment. Appl Health Econ Health Policy 2023; 21: 91‐107.
- 18. World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: WHO, 2021. https://www.who.int/publications/i/item/9789240030787 (viewed Aug 2024).
- 19. Goth C, Godehardt D, Godehardt E, et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod 2003; 18: 1959‐1966.
- 20. Björndahl L, Kirkman Brown J; Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in basic examination of human ejaculates. Fert Steril 2022; 117: 246‐251.
- 21. Wessel JA, Mochtar MH, Besselink DE, et al. Expectant management versus IUI in unexplained subfertility and a poor pregnancy prognosis (EXIUI study): a randomized controlled trial. Hum Reprod 2022; 37: 2808‐2816.
- 22. Lai S, Wang R, van Wely M, et al. IVF versus IUI with ovarian stimulation for unexplained infertility: a collaborative individual participant data meta‐analysis. Hum Reprod Update 2024; 30: 174‐185.
- 23. Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci 2022; 17: 75.
- 24. Au LS, Feng Q, Shingshetty L, et al. Evaluating prognosis in unexplained infertility. Fertil Steril 2024; 121(5): 717‐729.
- 25. Norman RJ. Prophecy, prediction, and prognosis — can we improve the advice we give on the chance of pregnancy and treatment options for infertility? Fertil Steril 2024; 121: 715‐716.
- 26. Bensdorp AJ, van der Steeg JW, Steures P, et al. A revised prediction model for natural conception. Reprod Biomed Online 2017; 34: 619‐626.






Abstract
Introduction: The 2024 Australian evidence‐based guideline for unexplained infertility provides clinicians with evidence‐based recommendations for the optimal diagnostic workup for infertile couples to establish the diagnosis of unexplained infertility and optimal therapeutic approach to treat couples diagnosed with unexplained infertility in the Australian health care setting. The guideline recommendations were adapted for the Australian context from the rigorous, comprehensive European Society of Human Reproduction and Embryology (ESHRE) 2023 Evidence‐based guideline: unexplained infertility, using the ADAPTE process and have been approved by the Australian National Health and Medical Research Council.
Main recommendations: The guideline includes 40 evidence‐based recommendations, 21 practice points and three research recommendations addressing:
Changes in assessment and management resulting from the guideline: This guideline refines the definition of unexplained infertility and addresses basic diagnostic procedures for infertility assessment not considered in previous guidelines on unexplained infertility. For therapeutic approaches, consideration of evidence quality, efficacy, safety and, in the Australian setting, feasibility, acceptability, cost, implementation and ultimately recommendation strength were integrated across multidisciplinary expertise and consumer perspectives in adapting recommendations to the Australian context by using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework, which had not been used in past guidelines on unexplained infertility to formulate recommendations. The Australian process also included an established data integrity check to ensure evidence could be trusted to guide practice. Practice points were added and expanded to consider the Australian setting. No evidence‐based recommendations were underpinned by high quality evidence, with most having low or very low quality evidence. In this context, research recommendations were made including those for the Australian context. The full guideline and technical report are publicly available online and can be accessed at https://www.monash.edu/medicine/mchri/infertility and are supported by extensive translation resources, including the free patient ASKFertility mobile application (https://www.askfertility.org/).