Clinical record
A 64‐year‐old man of European descent presented to a tertiary hospital in the Northern Territory with a four‐week history of fevers, headaches, coughing and dyspnoea.
His background included follicular lymphoma, for which he received maintenance obinutuzumab therapy every two months, having achieved remission the previous year. He had no other medical conditions. He had received two doses of the coronavirus disease 2019 (COVID‐19) vaccine, with the most recent dose 12 months prior and prophylactic tixagevimab–cilgavimab five months prior.
He was febrile at 39°C and tachypnoeic at 26 breaths per minute, with a blood oxygen saturation level of 97% on room air. On chest auscultation, there were bilateral inspiratory crepitations. Blood results revealed a raised C‐reactive protein concentration and a normal white cell count. Chest computed tomography scans showed bilateral pulmonary infiltrates with no pulmonary embolism. He was treated for sepsis secondary to bilateral pneumonia with piperacillin–tazobactam and vancomycin as per local guidelines. His nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) polymerase chain reaction (PCR) revealed a weak positive result, reflected by the high cycle threshold value of 36.9. This result prompted a search for an alternative pathogen.
During treatment with broad spectrum antibiotics, he had daily fevers higher than 38°C and a worsening blood oxygen saturation level of 88% on room air, correctable with 4 L of oxygen therapy. On Day 9 and 13, sputum samples were tested for SARS‐CoV‐2 (PCR). The resulting cycle threshold values of 32.0 and 23.7 respectively suggested active viral replication. These results prompted a ten‐day course of remdesivir and dexamethasone. Although he met the criteria for severe COVID‐19, baricitinib was not initiated due to an ongoing suspicion for concomitant bacterial infection. He rapidly defervesced but remained on 4 L oxygen therapy. On Day 23, within 48 hours of COVID‐19 treatment cessation, fevers higher than 38°C recurred and dyspnoea worsened. Interval chest imaging scans demonstrated progressive bilateral pulmonary infiltrates with widespread ground glass opacities.
Serial samples for SARS‐CoV‐2 PCR demonstrated decreasing cycle threshold values while off COVID‐19 treatments, suggesting recrudescence of COVID‐19 pneumonitis (Box). We re‐initiated treatment with remdesivir and dexamethasone, while awaiting whole genome sequencing (WGS), which identified the Omicron recombinant subvariant XBB.1.5. A literature review revealed studies demonstrating significantly reduced neutralising activity of anti‐spike protein monoclonal antibodies against subvariant XBB.1.5, including tixagevimab–cilgavimab.1
We escalated concerns to the Australian Society of Infectious Diseases Ozbug community, an online platform for infectious diseases physicians to share ideas and seek help with complex cases. The consensus was for combined therapies targeting viral suppression and the inflammatory response using antiviral agents, nirmatrelvir–ritonavir and remdesivir, and immunomodulatory agents including baricitinib and dexamethasone. As he was hypogammaglobulinaemic while on B‐cell depleting therapy, we also administered intravenous immunoglobulin (IVIG). Given the degree of iatrogenic immunosuppression in our already immunocompromised patient, we commenced prophylactic anti‐microbial therapies to mitigate the risk of opportunistic infections (Box).
During this treatment course, he defervesced at 48 hours and was weaned off oxygen. His levels of serum inflammatory markers and chest imaging scans demonstrated marked improvement. He was discharged with a negative SARS‐CoV‐2 PCR result on nasopharyngeal swab and has remained well in the community.
Discussion
We present a case of severe, prolonged COVID‐19 pneumonitis in an immunocompromised patient secondary to SARS‐CoV‐2 Omicron subvariant XBB.1.5. infection. Increased morbidity and mortality due to COVID‐19 is well described in patients suffering from an underlying haematological malignancy or receiving lymphocyte‐depleting therapy, both of whom may have attenuated vaccine‐induced humoral immunity.2,3
Subvariant XBB.1.5. is categorised as a variant of interest by the Communicable Diseases Genomics Network due to enhanced pathogenicity and transmissibility. Although reduced routine testing will likely underestimate the true incidence, subvariant XBB.1.5. accounted for 4.3% of variants of interest during a 28‐day period in November 2023.4
Treatment options are limited by a significant rate of viral escape to vaccine immune response and neutralising antibodies targeting the spike protein. With respect to IVIG preparations, there is evidence to suggest they may contain COVID‐19 neutralising antibodies. Our use of a fresh batch of IVIG theoretically increased the probability of neutralising antibodies to currently circulating variants. IVIG may have a role in treating COVID‐19 in lymphocyte‐depleted patients or those who are hypogammaglobulinaemic.5 More robust studies are needed to determine the best treatment options for COVID‐19 in immunocompromised patients. However, rapidly evolving variants present a challenge for researchers as ongoing efficacy of treatments is likely to be affected.
We also acknowledge the discordance in cycle threshold values between sputum and nasopharyngeal sampling. Sputum sampling is more sensitive for diagnosing COVID‐19 pneumonitis, and a higher sputum viral load correlates with increased disease severity.2,3,6 Where the diagnosis of COVID‐19 pneumonitis is suspected, especially in immunocompromised patients, a SARS‐Cov‐2 PCR test using a sputum sample should be undertaken. WGS also plays an important role in isolating the causative subvariant and directing subsequent management.
Lessons from practice
- In immunocompromised patients with COVID‐19, more intensive therapies and longer treatment durations may be required, although sufficient evidence is lacking.
- Immunocompromised patients are under‐represented in clinical trials, where optimal therapy is yet to be described.
- Omicron subvariant XBB.1.5. is considered a variant of interest due to concerning rates of viral escape compared with other circulating variants.
- Anti‐spike protein COVID‐19 vaccines and anti‐viral treatments such as tixagevimab–cilgavimab have limited efficacy against Omicron subvariant XBB.1.5.
- Where COVID‐19 pneumonitis is suspected, particularly in immunocompromised patients, obtaining sputum for SARS‐CoV‐2 PCR is important to confirm the diagnosis.
- Whole genome sequencing has a role to play in identifying variants of concern over more easily managed variants, particular in immunocompromised patients where results will affect treatment.
Box – Summary of treatment regimen and cycle threshold changes
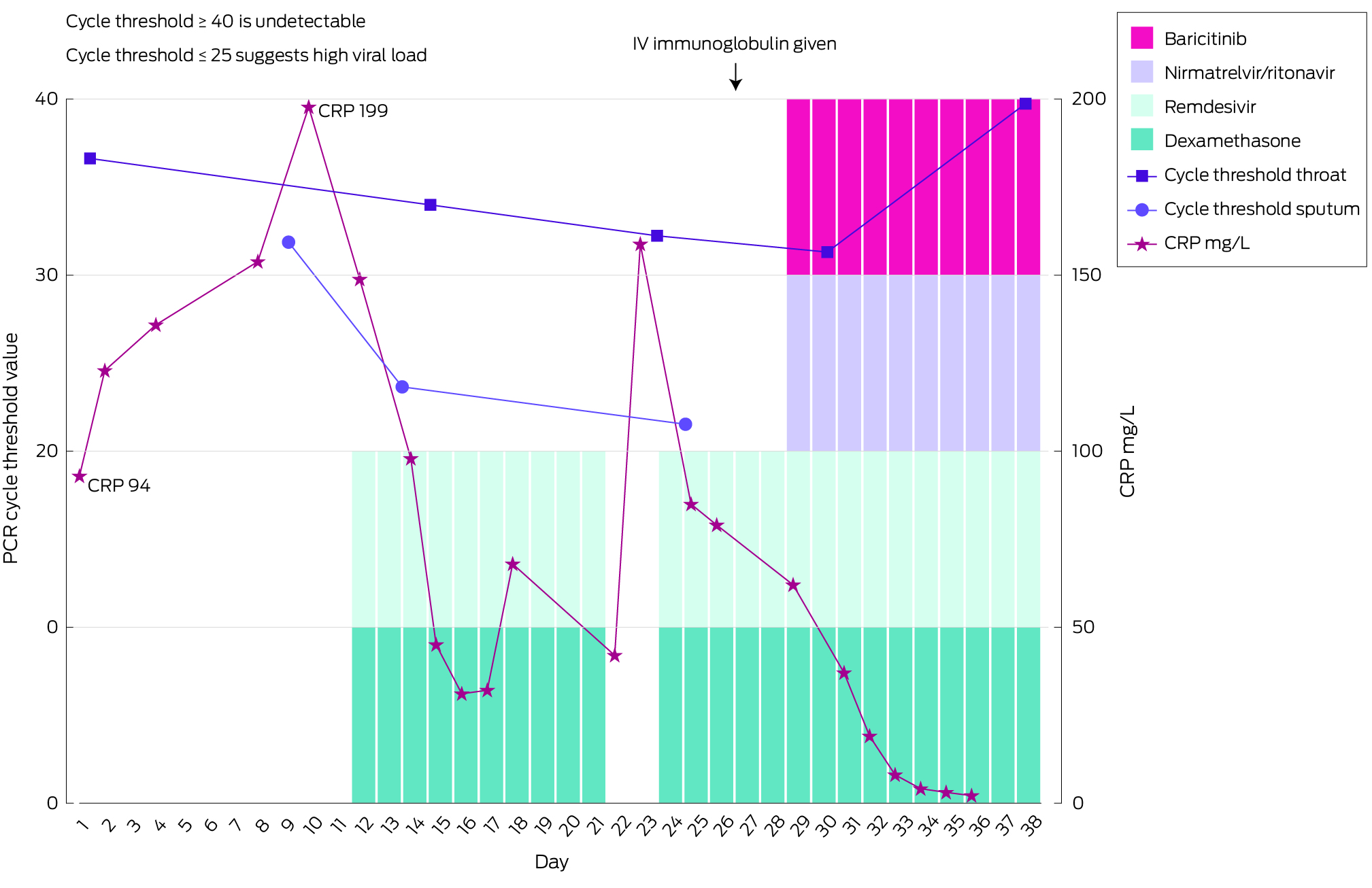
CRP = C‐reactive protein, IV = intravenous, PCR = polymerase chain reaction.COVID‐19 treatment: intravenous immunoglobulin, single dose 45 g; nirmatrelvir–ritonavir, 150 mg/100 mg twice daily for 10 days; baricitinib, 4 mg once daily for 14 days; remdesivir, intravenous 200 mg loading dose, followed by 100 mg once daily for 14 days; dexamethasone, 4 mg oral once daily for 15 days. Prophylactic therapies: liposomal amphotericin B, 200 mg thrice weekly; valganciclovir, 900 mg once daily; trimethoprim–sulfamethoxazole, 800 mg/160 mg once daily.
Provenance: Not commissioned; externally peer reviewed.
- 1. Uraki R, Ito M, Kiso M, et al. Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate. Lancet Infect Dis 2023; 23: 402‐403.
- 2. Liebers N, Speer C, Benning L, et al. Humoral and cellular responses after COVID‐19 vaccination in anti‐CD20‐treated lymphoma patients. Blood 2022; 139: 142‐147.
- 3. Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of covid‐19 vaccines in immunocompromised patients: systematic review and meta‐analysis. BMJ March 2, 2022: e068632; https://doi.org/10.1136/bmj‐2021‐068632.
- 4. Communicable Diseases Genomics Network. Variants of concern. 2023. https://www.cdgn.org.au/variants‐of‐concern (viewed Feb 2024).
- 5. Huygens S, Hofsink Q, Nijhof IS, et al. Hyperimmune globulin for severely immunocompromised patients hospitalized with Coronavirus disease 2019: a randomized, controlled trial. J Infect Dis 2023; 227: 206‐210.
- 6. Pagano L, Salmanton‐García J, Marchesi F, et al. Breakthrough COVID‐19 in vaccinated patients with hematologic malignancies: results from the EPICOVIDEHA survey. Blood 2022; 140: 2773‐2787.





Patient consent:
The patient provided written consent for publication.
No relevant disclosures.