Every year, about 1800 Australians die of hepatocellular carcinoma (HCC), the most common type of primary liver cancer.1
Aboriginal and Torres Strait Islander peoples of Australia (hereon respectfully referred to as First Nations Australians) are 2.5 times more likely to develop HCC and 1.4 times more likely to die from HCC than non‐Indigenous Australians.2
First Nations Australians with HCC have a 9% five‐year survival rate compared with 23% for non‐Indigenous Australians,2 and are half as likely to be diagnosed with early‐stage HCC and receive curative therapy.2 This is driven by First Nations Australians being adversely affected by social, cultural and commercial determinants of health stemming from colonisation, racism and remoteness.3
Chronic liver disease is the key cause of HCC and most chronic liver disease is preventable and treatable.4,5 First Nations Australians shoulder a disproportionate burden of chronic liver disease (Box 1).6 Alcohol‐related liver disease is a leading cause of HCC in all Australians, including First Nations Australians.2,7,8 The prevalence of hepatitis B and C is two‐ to three‐fold higher in First Nations Australians compared with non‐Indigenous Australians.2,5 The most prevalent hepatitis B genotype in remote First Nations communities (genotype C4) is associated with more aggressive liver disease and increased HCC risk compared with other genotypes.9,10 Obesity and type 2 diabetes, both leading risk factors for metabolic‐associated fatty liver disease, are twice as common in First Nations Australians than in non‐Indigenous Australians.3 Importantly, First Nations Australians are more likely to have multiple cofactors driving liver injury,2 warranting a multipronged approach to HCC prevention.
Methods
The two lead authors of this article, one First Nations Australian and one non‐Indigenous Australian, established a nationally representative, diverse group of four First Nations Australian and 14 non‐Indigenous Australian clinical and research leaders in the fields of HCC and chronic liver disease for the project. All authors have expertise in the provision of regional or remote models of HCC or chronic liver disease care, or both. First, an initial two‐hour virtual meeting was held, where authors shared their thoughts and responses to two main topics: i) identifying unmet needs in prevention, diagnosis and treatment of HCC in First Nations Australians; and ii) identifying opportunities to address these unmet needs. All authors shared key evidence and their experiences and perspectives, representing the differing epidemiology, health resourcing, policy and legislative contexts across all Australian states and territories. From this discussion, the lead authors developed a list of eight key action items using a positive, evidence‐based approach that prioritised First Nations‐led interventions and models of care. The aim was to retain action items that had 100% agreement across the author group; all eight action items were ratified by all authors.
Overcoming the challenges: steps for action
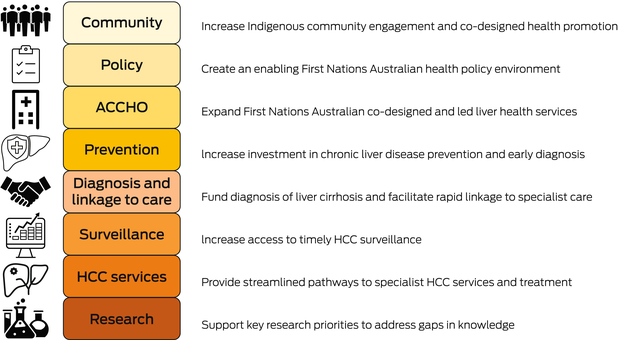
As a nationwide collaboration of First Nations and non‐Indigenous health workers and researchers in HCC, we identified eight key action measures, which are listed below, that will help address disparities in HCC incidence and mortality in First Nations Australians that should be urgently prioritised for investment (Box 2, Box 3, Box 4).
Increase Indigenous community engagement and co‐designed health promotion
Community engagement through partnership with First Nations Australians is the first step to improving HCC outcomes in Australia. The public health response to HCC in First Nations people should be determined and led by First Nations communities and Aboriginal Community Controlled Health Organisations (ACCHOs), supported by medical and community stakeholders. To sustain community engagement, we need more funding for effective First Nations co‐designed, culturally safe health education resources in First Nations languages and health promotion programs,11 Indigenous‐led civil society organisations that promote liver health, and the strengthening of the First Nations peer workforce.12
Stigma and discrimination are critical barriers to liver disease diagnosis in First Nations Australians, amplified by intersectionality with social, cultural and economic factors.3,13 National approaches to improving community and health worker education are needed to overcome systemic discrimination and entrenched health disparities for First Nations Australians.
Create an enabling Indigenous health policy environment
Historically, liver disease and HCC have not been included in Close the Gap initiatives,14 or the National Aboriginal and Torres Strait Islander health plan, despite being key drivers of premature mortality in First Nations Australians. For the first time, prevention and detection of primary liver cancer has been included in the National Aboriginal Community Controlled Health Services (NACCHO) and Royal Australian College of General Practitioners National guide to a preventive health assessment for Aboriginal and Torres Strait Islander People,15 an important first step. Currently, many liver care programs for remote areas are unsustainably funded through research grants. Liver disease and HCC measures should be included in national key performance indicators for Aboriginal and Torres Strait Islander primary health care and funding leveraged from the $230 million federal budget allocated to improve cancer outcomes in First Nations Australians.
Removing barriers to viral hepatitis test frequency, enabling reflex testing and the use of rapid point‐of‐care tests and dried blood spot testing would increase access to viral hepatitis testing for First Nations Australians.16 Governments should work closely with First Nations leaders to develop ambitious yet workable policy and legislation that focus on improved liver health for First Nations communities, such as improved alcohol policies and improved access and affordability of fresh unprocessed foods to reduce the risks of metabolic‐associated fatty liver disease.
Expand First Nations Australian co‐designed and led liver health services
ACCHOs are essential in providing culturally safe care. Convenience, acceptability and trusted relationships increase linkage and retention in care.16 Increased employment and training of Aboriginal and Torres Strait Islander health workers and peers builds a culturally safe health workforce and increases continuity of care on Country through greater staff retention. Expansion of the ACCHO health workforce should be leveraged for HCC models of care. However, a significant proportion of First Nations Australians do not access ACCHOs, therefore improving the cultural safety of health services more broadly will improve access for all First Nations Australians. Specialist nurse support for ACCHO health workers and general practitioners, outreach multidisciplinary liver services and telehealth models of hub‐and‐spoke specialist care will improve rapid specialist care access and uptake for First Nations Australians,2,5 particularly in regional and remote areas.
Increase investment in chronic liver disease prevention and early diagnosis
Offering screening for chronic liver disease to all First Nations Australians, or a “healthy liver check”, is vital to ensure timely identification and treatment of chronic liver disease. Although alcohol abstinence rates are higher in First Nations Australians than in non‐Indigenous Australians,17 active screening for harmful alcohol use and early linkage to First Nations community alcohol counselling and treatment services should be increased. High levels of hepatitis B vaccination coverage have been achieved in many remote communities;6,10 however, catch‐up programs for First Nations Australian adults, particularly in regional areas, are needed. Funding for Close the Gap infant mortality reduction should be harnessed for perinatal hepatitis B transmission prevention within a “triple elimination” strategy.18 Timely diagnosis and treatment of people with hepatitis B is proven to reduce HCC risk.19 Increased access to needle and syringe exchange programs, opiate agonist therapy programs and timely test‐and‐treat models of hepatitis C care are vital to prevent further transmission.20,21 Embedding chronic liver disease screening and management within existing metabolic syndrome health programs for First Nations Australians, such as type 2 diabetes or childhood obesity, would optimise resource allocation and cost‐effectiveness.
Fund early detection of cirrhosis and facilitate rapid linkage to specialist care
There are major disparities in access and funding for the tools used for cirrhosis diagnosis across Australia. Transient elastography is the non‐invasive international standard for diagnosis of cirrhosis;22 however, it remains unfunded by Medicare and there are no national access programs. Increased access to transient elastography is vital for all Australians; however, specific investment in portable transient elastography outreach services provided through ACCHOs is paramount for timely diagnosis of cirrhosis for First Nations Australians, particularly in regional and remote areas.
The fibrosis‐4 index and the aspartate aminotransferase to platelet ratio index are calculated using the results from routine blood tests and have a high negative predictive value for cirrhosis.22 Although not specifically validated in First Nations Australians, they provide a means of triaging those most in need of transient elastography and specialist assessment.22 Investment in health worker education programs to increase routine use of these simple tests in primary care should be prioritised. Other accurate but commercial blood test algorithms, such as Hepascore or Enhanced Liver Fibrosis test (Siemens Healthcare),22 would increase access to cirrhosis diagnosis in remote Australia, but remain unfunded through Medicare.
For those with cirrhosis, rapid linkage to specialist liver care improves survival. Investment in models of care that increase access is vital, such as nurse‐led care, telehealth hub‐and‐spoke models of care, and specialist outreach services.5,16,18
Increase access to timely HCC surveillance
HCC surveillance using six‐monthly liver ultrasounds is cost‐effective for improving early detection and survival in HCC.4,23,24 Uptake of HCC surveillance is low in First Nations Australians due to lack of awareness and access barriers, although uptake is high when offered on Country.16 Investment in novel models of HCC surveillance for remote Australia is urgently needed; for example, pilot outreach models of care delivered in partnership with the Royal Flying Doctors Service, greater training of local health workers in liver ultrasound, and exploration of new technologies, such as small portable ultrasounds.5
HCC risk scores using blood biomarkers could be a game changer to improve access to HCC surveillance in remote Australia. For example, the commercial GAAD score (gender, age, alfa‐fetoprotein, des‐carboxyprothrombin; Roche Diagnostics) has a high negative predictive value for HCC.25 Evaluation of accuracy and acceptability of HCC risk prediction scores for HCC surveillance in First Nations Australians should be prioritised, with fast‐tracked Medicare reimbursement if effective.
Finally, streamlined systems of HCC surveillance and recall that rapidly link communities to confirmatory imaging and multidisciplinary HCC care are essential. Peer navigators have been effectively used for other cancers in First Nations Australians.26
Provide streamlined pathways to specialist HCC services
HCC diagnosis and treatment access present a so‐called tyranny of distance for First Nations Australians, with distances from specialist care and radiology services contributing to shorter survival times in First Nations Australians in remote communities.13 Liver transplantation provides excellent outcomes for people with early‐stage HCC and impaired liver function;11 however, First Nations Australians are under‐represented in liver transplant recipients.27 Greater virtual access to specialist hospital HCC multidisciplinary team meetings should be promoted to local health workers to improve two‐way communication and training, enabling greater advocacy for First Nations Australians.
Most treatments for HCC are provided through tertiary hospitals, associated with significant cost, time off work for travel and disconnection from community for First Nations Australians. Outreach interventional radiology services for HCC through local hospital networks may improve access for First Nations people in remote areas. Systemic therapies offer First Nations Australians options for care on Country through community delivered treatments. Greater training of local health workers in recognising and managing potential complications will improve treatment outcomes. Greater efforts to offer First Nations Australians participation in HCC clinical trials are needed; teletrial initiatives have expanded trial access for people in remote settings.28
There is also a critical need for co‐designed palliative care services for First Nations Australians with HCC,29 supported by peers to promote uptake and improve coverage of culturally safe end‐of‐life care.
Support key research priorities
In Australia, most disease surveillance reports describe unspecified primary liver cancer.1 Accurate coding of liver cancer subtypes in Australian cancer data registries is paramount to track the effect of public health interventions on HCC incidence and mortality and should be prioritised. Understanding the barriers and enablers to uptake of HCC surveillance in First Nations people and reasons for late presentation and low treatment uptake are paramount to co‐design effective HCC surveillance and treatment programs that meet the needs of First Nations Australians.
Research priorities must be set and co‐led by First Nations Australians.30 Greater understanding of biological and epidemiological drivers of HCC; optimal HCC surveillance, diagnostic and treatment modalities; and effective, culturally safe health system solutions to improve HCC outcomes for First Nations Australians are urgently needed.
Funding for First Nations Australian‐led research should sustainably support meaningful community engagement and research capacity building.30 Importantly, it cannot be assumed that a model of care working in one community will translate effectively to another.31 Ongoing assessment should be embedded within HCC programs to ensure they are meeting the needs of First Nations Australians.
Conclusion
Although HCC prevalence and mortality remain unacceptably high in First Nations Australians, data from successful programs co‐led by First Nations Australians provide new hope for achieving greater parity in HCC outcomes. Now is the time to act in partnership with First Nations Australians to close the gap in liver disease outcomes and reduce preventable deaths from HCC.
Box 1 – Driving causes of chronic liver disease and hepatocellular carcinoma (HCC)
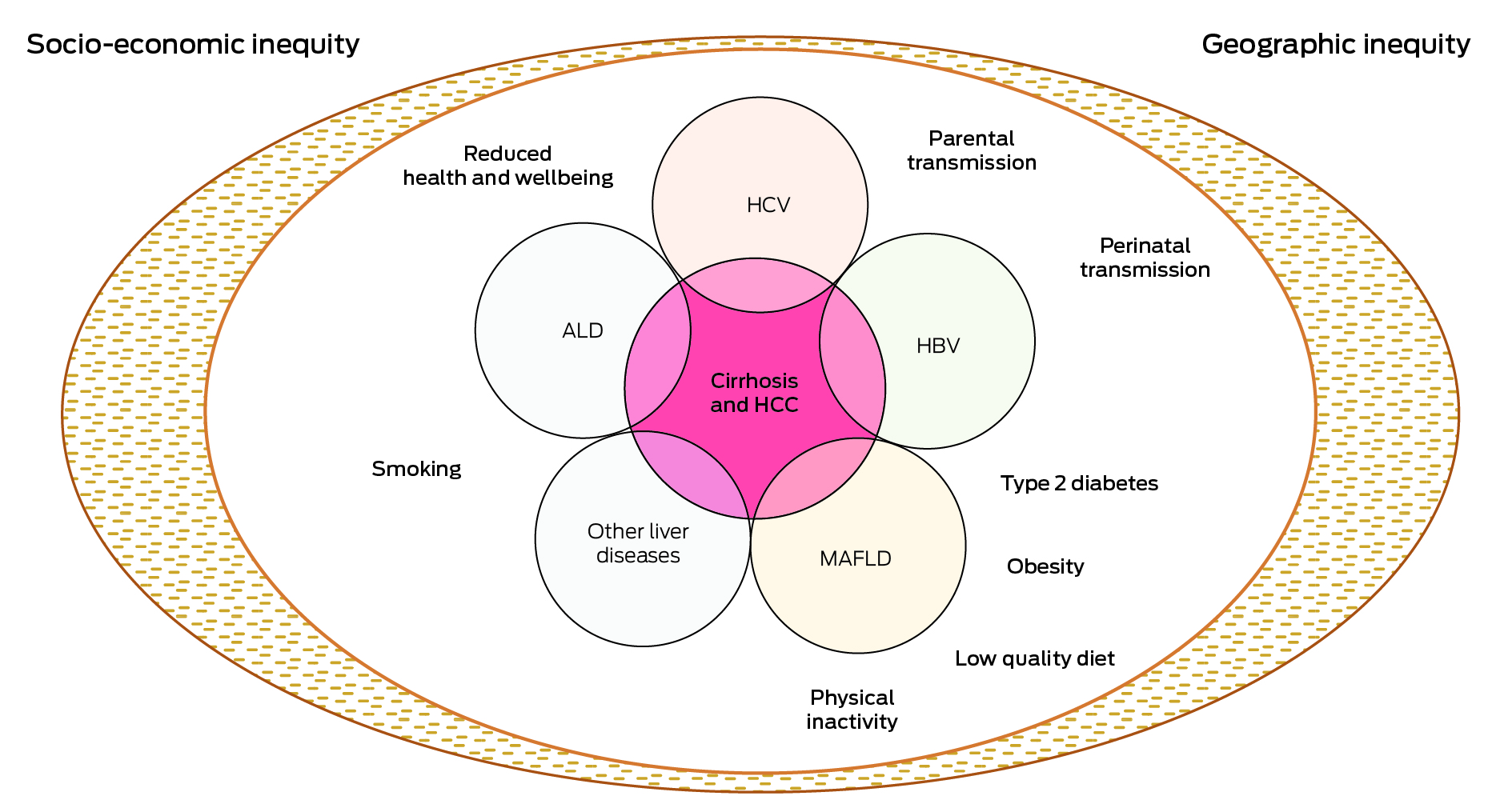
ALD = alcoholic liver disease; HBV = hepatitis B virus; HCV = hepatitis C virus; MAFLD = metabolic associated fatty liver disease. Most causes of chronic liver disease lead to HCC through the interim step of cirrhosis development. However, chronic hepatitis B infection and MAFLD may cause HCC in the absence of cirrhosis. Additionally, 30–40% of First Nations Australians have more than one risk factor for HCC.2
Box 2 – Hepatocellular carcinoma (HCC) action plan for First Nations Australians
|
Steps for action |
Aims |
Actions |
|||||||||||||
|
|
|||||||||||||||
|
Health promotion |
Increase Indigenous community engagement and culturally sensitive health promotion |
|
|||||||||||||
|
Policy |
Create an enabling Indigenous health policy environment |
|
|||||||||||||
|
Models of care |
Expand Indigenous co‐designed and led health services |
|
|||||||||||||
|
Prevention |
Increase investment in liver disease prevention and early diagnosis |
|
|||||||||||||
|
Risk identification |
Fund diagnosis of cirrhosis and facilitate rapid linkage to specialist care |
|
|||||||||||||
|
Surveillance |
Increase access to timely HCC surveillance |
|
|||||||||||||
|
Management |
Provide streamlined pathways to HCC services and treatment |
|
|||||||||||||
|
Research |
Support key research priorities to address gaps in knowledge |
|
|||||||||||||
|
|
|||||||||||||||
|
MBS = Medicare Benefits Schedule; NACCHO = National Aboriginal Community Controlled Health Services; TGA = Therapeutic Goods Administration. |
|||||||||||||||
Box 3 – Two‐, five‐ and ten‐year health policy action plan to reduce hepatocellular carcinoma (HCC) in First Nations Australians
|
Timeline |
Actions |
||||||||||||||
|
|
|||||||||||||||
|
2 years |
|
||||||||||||||
|
5 years |
|
||||||||||||||
|
10 years |
Achieve targets for HCC cascade of care*:
|
||||||||||||||
|
|
|||||||||||||||
|
MBS = Medicare Benefits Schedule; ACCHS = Aboriginal Community Controlled Health Service. * Would benefit all Australians, although should be prioritised for First Nations Australians. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Australian Institute of Health and Welfare. Liver cancer statistics. 2018. https://www.canceraustralia.gov.au/cancer‐types/liver‐cancer/statistics (viewed Mar 2024).
- 2. Wigg AJ, Narayana SK, Hartel G, et al. Hepatocellular carcinoma amongst Aboriginal and Torres Strait Islander peoples of Australia. EClinicalMedicine 2021; 36: 100919.
- 3. Australian Institute of Health and Welfare. Determinants of health for Indigenous Australians. Jul 2022. https://www.aihw.gov.au/reports/australias‐health/social‐determinants‐and‐indigenous‐health (viewed Mar 2024).
- 4. Lubel JS, Roberts SK, Strasser SI, et al. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Med J Aust 2020; 214: 475‐483. https://www.mja.com.au/journal/2021/214/10/australian‐recommendations‐management‐hepatocellular‐carcinoma‐consensus
- 5. Howell J, Ward JS, Davies J, et al. Hepatocellular carcinoma in Indigenous Australians: a call to action. Med J Aust 2021; 214: 201‐202. https://www.mja.com.au/journal/2021/214/5/hepatocellular‐carcinoma‐indigenous‐australians‐call‐action
- 6. Howell J, Pedrana A, Cowie BC, et al. Aiming for the elimination of viral hepatitis in Australia, New Zealand, and the Pacific Islands and Territories: where are we now and barriers to meeting World Health Organization targets by 2030. J Gastroenterol Hepatol 2019; 34: 40‐48.
- 7. Hong TP, Gow P, Fink M, et al. Novel population‐based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 2016; 63: 1205‐1212.
- 8. Flores JE, Hong T, Thompson AJ, et al. Metabolic‐associated fatty liver disease and alcohol‐related liver disease are leading causes of hepatocellular carcinoma: interim analysis of the HOMER‐2 study. J Gastroenterol Hepatol 2022; 37: 56.
- 9. Littlejohn M, Davies J, Yuen L, et al. Molecular virology of hepatitis B virus, sub‐genotype C4 in northern Australian Indigenous populations. J Med Virol 2014; 86: 695‐706.
- 10. Davies J, Li SQ, Tong SY, et al. Establishing contemporary trends in hepatitis B sero‐epidemiology in an Indigenous population. PLoS One 2017; 12: e0184082.
- 11. Davies J, Bukulatjpi S, Sharma S, et al. Development of a culturally appropriate bilingual electronic app about hepatitis B for Indigenous Australians: towards shared understandings. JMIR Res Protoc 2015; 4: e70.
- 12. Hosking K, De Santis T, Vintour‐Cesar E, et al. “The most culturally safe training I've ever had”: the co‐design of a culturally safe Managing hepatitis B training course with and for the Aboriginal health workforce of the Northern Territory of Australia. BMC Health Serv Res 2023; 23: 935.
- 13. Clark PJ, Stuart KA, Leggett BA, et al. Remoteness, race and social disadvantage: disparities in hepatocellular carcinoma incidence and survival in Queensland, Australia. Liver Int. 2015; 35: 2584‐2594.
- 14. Australian Government. National agreement on closing the gap. Closing the gap in partnership, July 2020. https://www.closingthegap.gov.au/sites/default/files/files/national‐agreement‐ctg.pdf (viewed July 2024).
- 15. National Aboriginal Community Controlled Health Organisation and The Royal Australian College of General Practitioners. National guide to a preventive health assessment for Aboriginal and Torres Strait Islander people. 3rd ed. East Melbourne: RACGP, 2018.
- 16. Hla TK, Bukulatjpi SM, Binks P, et al. A “one stop liver shop” approach improves the cascade‐of‐care for Aboriginal and Torres Strait Islander Australians living with chronic hepatitis B in the Northern Territory of Australia: results of a novel care delivery model. Int J Equity Health 2020; 19: 64.
- 17. Australian Bureau of Statistics. National Aboriginal and Torres Strait Islander health survey. Dec 2019. https://www.abs.gov.au/statistics/people/aboriginal‐and‐torres‐strait‐islander‐peoples/national‐aboriginal‐and‐torres‐strait‐islander‐health‐survey/latest‐release (viewed Mar 2024).
- 18. Howell J, Pedrana A, Schroeder SE, et al. A global investment framework for the elimination of hepatitis B. J Hepatol 2021; 74: 535‐549.
- 19. Lubel JS, Strasser SI, Thompson AJ, et al. Australian consensus recommendations for the management of hepatitis B. Med J Aust 2022; 216: 478‐486. https://www.mja.com.au/journal/2022/216/9/australian‐consensus‐recommendations‐management‐hepatitis‐b
- 20. Pedrana A, Howell J, Scott N, et al. Global hepatitis C elimination: an investment framework. Lancet Gastroenterol Hepatol 2020; 5: 927‐939.
- 21. Cooke GS, Andrieux‐Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019; 4: 135‐184.
- 22. European Association for the Study of the Liver. EASL clinical practice guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis ‐ 2021 update. J Hepatol 2021; 75: 659‐689.
- 23. Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost‐effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol 2020; 115: 1642‐1649.
- 24. Cancer Council Australia. Clinical practice guidelines for hepatocellular carcinoma surveillance for people at high risk in Australia. 2023 [website]. https://www.cancer.org.au/clinical‐guidelines/liver‐cancer/hepatocellular‐carcinoma (viewed Mar 2024).
- 25. Best J, Bechmann LP, Sowa JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020; 18: 728‐735.
- 26. Bernardes CM, Martin J, Cole P, et al. Lessons learned from a pilot study of an Indigenous patient navigator intervention in Queensland, Australia. Eur J Cancer Care (Engl) 2018; 27. https://doi.org/10.1111/ecc.12714.
- 27. Australia and New Zealand liver and intestinal transplant registry. 34th Annual report ANZLITR, report on liver and intestinal transplantation activity to 31/12/2022. https://www.anzlitr.org/wp‐content/uploads/2024/04/ANZLITR_34th_Annual.pdf (viewed July 2024).
- 28. Sabesan S, Poxton M. Health equity in clinical trials for regional, rural and First nations communities: need for networked clinical trial system, through a values and purpose‐aligned system culture. Aust J Rural Health 2024; 32: 588‐591.
- 29. Panozzo S, Bryan T, Mason T, et al. Bridging cultures in palliative care: a qualitative study of the care of Indigenous Australians with advanced illness. Palliat Med 2023; 37: 498‐507.
- 30. Morris BA, Anderson K, Cunningham J, Garvey G. Identifying research priorities to improve cancer control for Indigenous Australians. Public Health Res Pract 2017; 27: 2741735.
- 31. D'Aprano A, Lloyd‐Johnson C, Cameron D, et al. Trusting relationships and learning together: a rapid review of Indigenous reference groups in Australian Indigenous health research. Aust N Z J Public Health 2023; 47: 100051.






Open access:
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Jessica Howell's salary is supported by an NHMRC Investigator Fellowship, NHMRC Program grant, Burnet Institute Program grant and University of Melbourne strategic grant. We gratefully acknowledge First Nations Australians as the original custodians of this land, who have generously shared their wisdom and stories that led to and informed this work.
Jessica Howell has received speaker fees and participated in advisory boards for Eisai, Astra Zeneca, Roche and Gilead; and received competitive grant funds from Gilead Sciences and Eisai. Troy Combo has participated in an advisory board for Astra‐Zeneca. Paula Binks has participated in advisory boards for Eisai and Astra‐Zeneca. Kylie Bragg has participated in an advisory board for Astra‐Zeneca. Kate Muller has participated in an advisory board for Astra‐Zeneca. Alan Wigg has participated in advisory boards for Eisai. Jacob George has participated in advisory boards and received honoraria for talks from Novo Nordisk, Astra‐Zeneca, Roche, BMS, Pfizer, Cincera, Pharmaxis, Boehringer Mannheim. Stuart Roberts has participated in advisory boards for Eisai, Astra‐Zeneca and Roche.