The emergence of novel monoclonal antibodies targeting amyloid‐β (mABs) has generated community anticipation around treatments that may slow the progression of Alzheimer disease. Some of these antibodies have been approved by the United States Food and Drug Administration and are under review in Australia. However, their efficacy needs to be carefully balanced against treatment risks and burden. Physicians caring for patients with Alzheimer disease will require new skills when considering these competing notions. This is challenging as clinical experience is limited, and the information necessary for thoughtful decision making is often not presented in a patient‐focused manner. In this perspective article, written by clinicians and people impacted by dementia, we highlight recent data regarding mABs and discuss the impact within a patient‐centric framework. Finally, we provide guidance on how to convey this information to individuals with Alzheimer disease and their carers and families.
Clinical trials of anti‐amyloid‐β therapy
Phase 3 trials of three intravenous mABs (aducanumab, EMERGE and ENGAGE;1 lecanemab, CLARITY‐AD;2 and donanemab, TRAILBLAZER‐ALZ 23), recruited individuals aged between 50 and 90 years with mild cognitive impairment or early Alzheimer disease dementia (Box 1). Importantly, inclusion required demonstration of elevated amyloid‐β (Aβ) either through positron emission tomography (PET) scans or cerebrospinal fluid analysis. The three mABs showed strong biological efficacy in Aβ removal, achieving a 60–85% reduction over 18 months, with up to 80% of participants rendered “amyloid‐negative” (as measured by Aβ‐PET scan).1,3,4
Contrastingly, the observed clinical benefits have been modest. Clinical effects are typically evaluated by quantifying the impact of Alzheimer disease on cognition and function, using tools such as the Clinical Dementia Rating (CDR) scale and the Integrated Alzheimer's Disease Rating Scale (iADRS).5,6 The CDR is scored across six domains, with higher values representing more impairment, and may be summed to form the CDR‐Sum of Boxes (CDR‐SB) score (0 to 18).5 Similarly, the iADRS is a composite measure of ability (scored 0 to 144), with a lower numerical value representing greater impairment.6
CDR was used to determine if treatment mitigated Alzheimer disease progression. Although the aducanumab trials were halted early due to futility analysis of interim data, subsequent analysis of the EMERGE trial demonstrated a 22% slowing of cognitive decline with high dose aducanumab compared with placebo (CDR‐SB adjusted mean difference of ‐0.39 with aducanumab versus placebo).1 Lecanemab and donanemab demonstrated a 27% and 36% slowing with a CDR‐SB difference of ‐0.45 and ‐0.67 respectively.2,3 With donanemab, treatment also halted disease progression at one year (as indicated by a stable CDR‐SB) in 47% of participants (compared with 29% with placebo).
Overall, these mABs resulted in a smaller decline of the CDR‐SB by about 0.5 over 18 months. This effect may be conceptualised as both an absolute change in the outcome measure at a specific time point (outcome‐based perspective) and as a time‐based delay to a certain clinical outcome (time‐based perspective, Box 2). With an outcome‐based perspective, a CDR‐SB reduction by 0.5 in a person with early Alzheimer disease would generally imply the presence of slight, rather than moderate, impairment in a single domain, with preservation of independence. For example, in the memory domain, this would equate to “slight forgetfulness with partial recollection of events” rather than “moderate memory loss, more marked for recent events.”5 The retained function with donanemab over 18 months represents a savings of about 50–75 hours of carer time over a period of 18 months.7 Such differences would likely be considered significant for individuals as well as their carers and families.8 Using a time‐based perspective, the CDR‐SB difference at 18 months equates to about 4.5 months of delayed progression, translating to a 25% slowing of disease.8 This degree of slowing is also considered a meaningful outcome for those with early Alzheimer disease.9 It is important to note that the reported outcomes represent the average benefit. As the understanding of factors influencing individual treatment response grows, including from phase 4 studies and real‐world experience, it may be possible to identify specific patients in which benefits may be larger.
Risks of anti‐amyloid‐β therapy
Balanced against the benefits are the risks of therapy, with amyloid‐related imaging abnormalities (ARIA) and infusion‐related reactions being the most important adverse effects. The results have shown that mABs were generally well tolerated with low rates of treatment discontinuation: 6.9% with lecanemab and 13.1% with donanemab, compared with 2.9% and 4.3% with placebo respectively. Over 70% of infusion‐related reactions did not require treatment interruptions or responded quickly to symptomatic management.
Amyloid‐related imaging abnormalities
ARIA, which include intracerebral oedema/effusion (ARIA‐E) and haemorrhage (ARIA‐H), are a key risk of mAB therapy.10 Although the precise mechanism is not understood, ARIA are thought to arise secondary to mAB‐mediated Aβ clearance from brain parenchyma and vasculature, resulting in compromised blood–brain barrier integrity with leaking of proteinaceous (ARIA‐E) and haem products (ARIA‐H).11
As ARIA is an imaging‐based phenomenon, regular brain magnetic resonance imaging (MRI) scans were included in the trials (four in the first year and one at the end of the treatment period for lecanemab and donanemab). ARIA‐E was seen at higher rates with mABs (12.6–34.8% of participants) compared with placebo (1.7–2.1%), with most cases occurring early in the treatment period.1,2,3,12 Of the ARIA‐E cases, 25% exhibited symptoms, including headache, confusion, seizures and visual disturbances. ARIA‐E usually resolved within 4 months of detection and did not lead to treatment discontinuation in mild cases. ARIA‐H was observed at similar rates to and often coinciding with ARIA‐E, and most patients were asymptomatic for cerebral microbleeds or superficial siderosis detected by MRI scans. A higher proportion of individuals with the apolipoprotein E (APOE) ε4 allele developed ARIA, with increased incidence seen with ε4 homozygosity compared with heterozygosity. There were 3 treatment‐related deaths (0.4%) with donanemab (compared with 1 with placebo) related to serious ARIA, whereas none were observed with aducanumab or lecanemab.
Management of ARIA
The development of ARIA did not necessarily result in treatment discontinuation, with less than 3.5% of ARIA cases in donanemab‐treated participants requiring cessation due to severe symptoms or progressive imaging findings.3 The trials demonstrated a safe paradigm of treatment continuation for asymptomatic ARIA‐E and for limited ARIA‐H. Reassuringly, ARIA censoring analyses demonstrated no significant change in functional outcomes, suggesting that mAB‐related side effects do not nullify clinical benefit.2,3
Treatment requirements and monitoring
Time commitments are considerable with mABs, involving fortnightly or monthly clinic visits that include a 1‐hour intravenous infusion in addition to pre‐ and post‐infusion observations, for up to 18 months. This is in addition to the assessments required for mAB eligibility, such as confirming elevation of Aβ (either by lumbar puncture or PET scan) and of tau (with tau PET scan for donanemab). Of note, applying trial inclusion and exclusion criteria to selected clinical cohorts in the United States suggests that between 5 and 20% of real‐world patients with mild cognitive impairment or early Alzheimer disease dementia would be eligible for mAB therapy, with most excluded due to medical comorbidities.13 It is expected that a similar proportion of Australian patients would be eligible, although current studies are lacking. Access to and uptake of lumbar punctures and amyloid/tau PET scans vary considerably between regions and may be a significant barrier for patients, potentially further restricting the number of eligible patients.
Some appropriate‐use guidelines recommend at least 3 brain MRI scans for ARIA surveillance within the first year of treatment.14,15 Further imaging may be required for ARIA management. In addition, APOE genotyping is recommended in some guidelines to more accurately predict ARIA risk. However, the implications of such genetic information for patients and family members may not be trivial, and most memory clinics do not have access to genetic counsellors to support the communication of this information.
Guidelines for how to assess patients for mAB treatment, as well as administering therapy, have not been established in Australia, although there may be clarity after review by regulatory bodies such as the Therapeutics Goods Administration.16,17 Coordinated efforts will be needed between general practitioners, nursing and allied health clinicians, specialists and health care systems with dementia prevention and treatment clinics. Models of care that minimise health disparities between rural/regional and metropolitan centres are essential.
Discussing trial data with patients
From a patient, carer and family perspective, comprehending the uncertainty surrounding benefit and risk may be daunting. Concepts such as hazard ratios and relative risk can confuse the actual effect on the patient as these concepts are not intuitive. Instead, use of precise language, absolute risks, and graphics such as icon arrays to depict risk are recommended (Box 3).18 Understanding how trial participants compare in terms of demographics, comorbidities, and disease stage may be facilitated using summarised trial baseline data as shown in Box 1.
The minimal clinically important difference (MCID) construct may help evaluate whether the benefits demonstrated in trials are meaningful for individual patients. This construct quantifies the smallest change in outcome that a patient would consider worthwhile.19 The MCID is specific to the outcome tool and the disease stage. For example, the MCID for CDR‐SB was estimated to be 0.54, 0.98 and 1.63 over a one‐year period for an individual with no cognitive impairment, mild cognitive impairment, and early Alzheimer disease respectively.20 It may appear that the mAB trials fail to meet this threshold, but these values indicated meaningful thresholds for within‐patient progression, and not group‐level differences.8,21 A personalised assessment of MCID (Box 2) may help frame whether mAB treatment would be beneficial. It is important to note that individual assessments of trade‐offs between benefits and risks of Alzheimer disease treatments are heterogeneous and influenced by a variety of factors; this individual variability should be incorporated into decision‐making discussions.22,23 For example, an individual that considers a CDR‐SB change of 1.5 over 18 months as their personal MCID and tolerates an increased risk for symptomatic oedema may perceive benefit from donanemab (as treatment reduced the CDR‐SB adjusted mean change from 1.84 to 1.16).
With these factors in mind, an individualised discussion of mABs with any patient should include:
- identifying how closely the patient overlaps with clinical trial participants, including inclusion and exclusion criteria;
- listing additional testing and eligibility requirements, including associated financial and time costs;
- illustrating the average and range of mAB benefits using functional and outcome changes with patient‐specific examples;
- listing typical clinical and imaging safety monitoring procedures, including associated financial and time costs;
- specifying mAB‐related side effects and their potential implications on treatment continuation and overall health and mortality; and
- making a patient‐centred shared decision regarding appropriateness of mAB therapy with ongoing multidisciplinary support for patients, carers and families.
Concluding remarks and future perspectives
Advances in anti‐amyloid therapy offer hope for individuals with Alzheimer disease, along with their carers and families. A careful approach is needed to discuss these treatments with patients, especially considering the associated risks. Whether patients perceive treatments as worthwhile considering therapy burden is a personal decision that may draw on group‐level findings from clinical trials and an individual application of the MCID. Although there are risks associated with treatment, the benefits are promising. Moreover, the field is advancing quickly with mABs being tested for dementia prevention in preclinical Alzheimer disease and new blood‐based biomarkers that may become part of the eligibility assessment process.24,25 Progress in finding effective and safe disease‐modifying therapies is offering optimism in the fight against Alzheimer disease.
Box 1 – Comparison of recent anti‐amyloid‐β antibody trials
|
|
Aducanumab: EMERGE1 (high dose) |
Lecanemab: CLARITY‐AD2 |
Donanemab: TRAILBLAZER‐ALZ 23 (low/medium tau group) |
||||||||||||
|
|
|||||||||||||||
|
Total number of participants |
1095 |
1795 |
1182 |
||||||||||||
|
Eligibility criteria |
50–85 years old |
50–90 years old |
60–85 years old |
||||||||||||
|
MCI or mild dementia |
MCI or mild dementia |
MCI or mild dementia |
|||||||||||||
|
PET Aβ+ |
PET or CSF Aβ+ |
PET Aβ + and tau PET+ |
|||||||||||||
|
MMSE ≥ 24 |
MMSE ≥ 22 |
MMSE 20–28 |
|||||||||||||
|
Mean MMSE |
26.3 ± 1.7 (treatment) |
25.5 ± 2.2 (treatment) |
23.1 ± 3.6 (treatment) |
||||||||||||
|
26.4 ± 1.8 (placebo) |
25.6 ± 2.2 (placebo) |
22.8 ± 3.8 (placebo) |
|||||||||||||
|
Infusion schedule |
Every 4 weeks for 76 weeks |
Every 2 weeks for 72 weeks |
Every 4 weeks for 72 weeks |
||||||||||||
|
ARIA‐E |
|
|
|
||||||||||||
|
All cases |
34.8% (treatment) |
12.6% (treatment) |
23.6% (treatment) |
||||||||||||
|
0.4% (placebo) |
1.7% (placebo) |
2.2% (placebo) |
|||||||||||||
|
Symptomatic cases |
24.2% (treatment)* |
2.8% (treatment) |
6.2% (treatment) |
||||||||||||
|
10.3% (placebo)* |
0% (placebo) |
0.2% (placebo) |
|||||||||||||
|
By APOE ε4 (treatment only) |
43.1% (carriers) |
15.8% (carriers) |
62.8% (carriers)† |
||||||||||||
|
17.9% (non‐carriers) |
5.4% (non‐carriers) |
15.7% (non‐carriers)† |
|||||||||||||
|
ARIA‐H |
20.0% (treatment) |
17.3% (treatment) |
30.7% (treatment) |
||||||||||||
|
6.8% (placebo) |
9.0% (placebo) |
13.8% (placebo) |
|||||||||||||
|
CDR‐SB adjusted mean difference v placebo at 18 months (95% CI) |
‐0.39 (‐0.69 to ‐0.09); P = 0.12 |
‐0.45 (‐0.67 to ‐0.23); P < 0.001 |
‐0.67 (‐0.95 to ‐0.40); P < 0.001 |
||||||||||||
|
22% slowing |
27% slowing |
36% slowing |
|||||||||||||
|
|
|||||||||||||||
|
Aβ = amyloid‐β; ARIA‐E = amyloid‐related imaging abnormalities with oedema/effusions; ARIA‐H = amyloid‐related imaging abnormalities with haemorrhage; APOE ε4 = apolipoprotein E ε4 allele; CDR‐SB = Clinical Dementia Rating Sum of Boxes; CI = confidence interval; CSF = cerebrospinal fluid; MCI = mild cognitive impairment; MMSE = Mini Mental State Examination; PET = positron emission tomography. * Symptomatic case data from combined EMERGE and ENGAGE data.12 † Data from donanemab combined tau group. |
|||||||||||||||
Box 2 – Illustrative progression of functional impairment over time with and without treatment
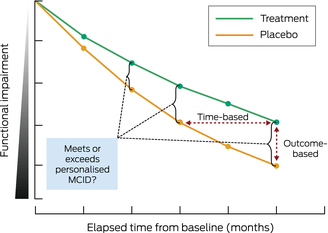
Treatment (green line) demonstrates slower disease progression compared with placebo (orange line) over time from starting the treatment/placebo (baseline). Functional assessments, as measured at various time points by outcome tools such as Clinical Dementia Rating and Integrated Alzheimer Disease Rating Scale, allow for time‐based (horizontal double arrow) and outcome‐based (vertical double arrow) perspectives. Determining when a personalised minimal clinically important difference (MCID) for an individual is met or exceeded is possible with the use of outcome tool metrics combined with a graphical representation of disease progression.
Box 3 – Outcomes with donanemab therapy compared with placebo in patients with mild cognitive impairment and mild dementia due to Alzheimer disease
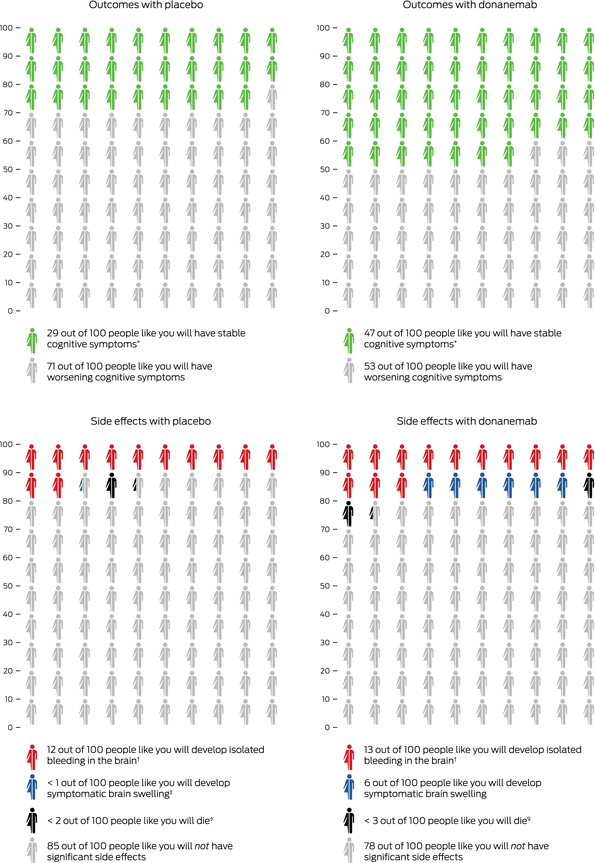
Icon array representation of TRAILBLAZER‐ALZ 2 trial3 for donanemab (low/medium tau group). Numbers indicate how many people will experience a given outcome when treated with placebo or donanemab over a 12‐month period for outcome (top graphs) or an 18‐month period for side effects (bottom graphs). * Outcome based on unchanged Clinical Dementia Rating Sum of Boxes score at 12 months, compared with baseline. † Value based on combined tau group data. ‡ The value of “< 1” is equal to 0.2. § “< 2” is equal to 1.3. ¶ “< 3” is equal to 2.1.
Provenance: Not commissioned; externally peer reviewed.
- 1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis 2022; 9: 197‐210.
- 2. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med 2023; 388: 9‐21.
- 3. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA 2023; 330: 512‐527.
- 4. Perneczky R, Jessen F, Grimmer T, et al. Anti‐amyloid antibody therapies in Alzheimer's disease. Brain 2023; 146: 842‐849.
- 5. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412‐2414.
- 6. Wessels AM, Siemers ER, Yu P, et al. A combined measure of cognition and function for clinical trials: the integrated Alzheimer's Disease Rating Scale (iADRS). J Prev Alzheimers Dis 2015; 2: 227‐241.
- 7. Wessels AM, Dennehy EB, Dowsett SA, et al. Meaningful clinical changes in Alzheimer disease measured with the iADRS and illustrated using the donanemab TRAILBLAZER‐ALZ study findings. Neurol Clin Pract 2023; 13: e200127.
- 8. Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement 2023; 19: 2730‐2736.
- 9. Insel PS, Weiner M, Mackin RS, et al. Determining clinically meaningful decline in preclinical Alzheimer disease. Neurology 2019; 93: e322‐e33.
- 10. Barakos J, Purcell D, Suhy J, et al. Detection and management of amyloid‐related imaging abnormalities in patients with Alzheimer's disease treated with anti‐amyloid beta therapy. J Prev Alzheimers Dis 2022; 9: 211‐220.
- 11. Hampel H, Elhage A, Cho M, et al. Amyloid‐related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain 2023; 146: 4414‐4424.
- 12. Salloway S, Chalkias S, Barkhof F, et al. Amyloid‐related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol 2022; 79: 13‐21.
- 13. Pittock RR, Aakre J, Castillo AM, et al. Eligibility for anti‐amyloid treatment in a population‐based study of cognitive aging. Neurology 2023; 101: e1837‐e1849.
- 14. Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis 2022; 9: 221‐230.
- 15. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis 2023; 10: 362‐377.
- 16. Therapeutic Goods Administration. Leqembi Eisai Australia Pty Ltd 2023. https://www.tga.gov.au/resources/prescription‐medicines‐under‐evaluation/leqembi‐eisai‐australia‐pty‐ltd (viewed Dec 2023).
- 17. Therapeutic Goods Administration. Kisunla Eli Lilly Australia Pty Ltd 2023. https://www.tga.gov.au/resources/prescription‐medicines‐under‐evaluation/kisunla‐eli‐lilly‐australia‐pty‐ltd (viewed Dec 2023).
- 18. Visser LNC, Minguillon C, Sanchez‐Benavides G, et al. Dementia risk communication. A user manual for Brain Health Services‐part 3 of 6. Alzheimers Res Ther 2021; 13: 170.
- 19. Cummings J. Meaningful benefit and minimal clinically important difference (MCID) in Alzheimer's disease: Open peer commentary. Alzheimers Dement (N Y) 2023; 9: e12411.
- 20. Andrews JS, Desai U, Kirson NY, et al. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement (N Y) 2019; 5: 354‐363.
- 21. Van Dyck CH, O'Dell RS, Mecca AP. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement (N Y) 2023; 9: e12388.
- 22. Roldan Munoz S, de Vries ST, Lankester G, et al. Preferences about future Alzheimer's disease treatments elicited through an online survey using the threshold technique. J Prev Alzheimers Dis 2023; 10: 756‐764.
- 23. Dementia Australia. Promising results of new Alzheimer's drug published – early diagnosis is key 2023. https://www.dementia.org.au/media‐centre/media‐releases/promising‐results‐new‐alzheimers‐drug‐published‐early‐diagnosis‐key (viewed Dec 2023).
- 24. Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer's disease in clinical practice and trials. Nat Aging 2023; 3: 506‐519.
- 25. Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3‐45 Study: design of a prevention trial for Alzheimer's disease. Alzheimers Dement 2023; 19: 1227‐1233.






Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
This work was supported by the Melbourne Genomics Health Alliance Genomics Immersion Fellowship (to Oneil Bhalala). No role of the funding source in preparation or publication of this article. We would like to acknowledge Julie Smith for her valuable contributions in reviewing and editing this perspective article.
No relevant disclosures.