|
Research
Colorectal cancer after open-access colonoscopy: a community and
case survey
John Croese
MJA 1999; 170: 251-254
For editorial comment, see Bolin & Korman
|
Abstract |
Objective: To evaluate whether colonoscopy
protects against subsequent colorectal cancer (CRC).
Design: Case and population survey.
Setting: Townsville region in northern Queensland,
between mid 1985 and January 1998.
Subjects: All 8430 patients who underwent 11 148
colonoscopies performed by the author at the Mater Private Hospital
(a community-based open-access colonoscopy service) between July
1985 and December 1996; those who were subsequently diagnosed with
CRC, to January 1998; and all 476 residents diagnosed with colorectal
cancer between 1994 and 1997.
Main outcome measures: Age-standardised CRC incidence
for 1994-1997 for patients who had had a previous colonoscopy and for
the remaining community; Dukes' grade of CRCs.
Results: For people aged 50 years and over, the incidence of CRC
was significantly lower among those who had had a previous
colonoscopy than in the remaining community (1.14 versus 2.31 per
1000 patient-years; P = 0.0046). For people aged 35-49 years,
the incidence was 0.35 versus 0.31 per 1000 patient-years (P =
0.86). Thirty-one CRCs developed in 29 people who had had previous
colonoscopy; only five of these CRCs were graded Dukes C, with none
graded Dukes D. In contrast, almost half the CRCs in the rest of the
community were graded Dukes C or D (P < 0.001). All but one of
those diagnosed with CRC on repeat colonoscopy had risk factors
(personal or family history), and 23 of the CRCs were subclinical,
with 20 being diagnosed during surveillance colonoscopy.
Conclusions: Patients aged over 50 years who had
previously undergone a colonoscopy and ensuing treatment were less
likely to be diagnosed with CRC than otherwise expected.
Surveillance colonoscopy led to diagnosis of CRCs with lower Dukes
grades.
|
| | Introduction |
Colorectal cancer (CRC) is the most common internal malignancy and
the second most common cause of death from cancer in Australia. CRC
incidence in Australia is similar to that in other developed
countries,1 but in the US both incidence
and mortality are now decreasing.2 This change has been
attributed to removal of premalignant polyps, detection of
proportionally more early lesions by colonoscopy, and more
effective treatment.3 Strategies based on
colonoscopic surveillance and targeting people at increased risk of
developing CRC are being promoted.3-5 However, colonoscopy,
particularly in community-based, open-access practice, has not
been shown to reduce CRC mortality. Indeed, although the rate of
colonoscopies in Australia has increased fourfold since
1984, the incidence of CRC in New South Wales between 1973 to 1992
continued to increase by 2% per year in men and 0.9% per year in
women.6 This study aimed to evaluate whether colonoscopy protects against
subsequent CRC by comparing CRC incidence and pathological grading
between people who have had a previous colonoscopy and the rest of the
population in a geographically isolated area.
|
| |
Methods |
| |
Setting |
The study was set in Townsville and the surrounding region (defined by
the postcodes 4804-4822, 4849 and 4850; Figure
1). Townsville is a regional centre in northern Queensland that
provides centralised health services, including colonoscopy, for a
population of 198 000 dispersed over 200 000 km2. The nearest alternative
colonoscopy services are located at Cairns and Mackay, 400 km
distant.
| |
Colonoscopy patients |
Subjects were patients who underwent colonoscopy performed by
myself at the Mater Private Hospital, Townsville. All patients who
underwent colonoscopy between July 1985 and December 1996 were
identified, most from the hospital's detailed computerised
records, but some of those examined between 1985 and 1990 from a
hospital work ledger which gave only surname and given name.
In addition, detailed demographic and clinical information was
collected prospectively in a procedural database for all
colonoscopy patients from April 1994 to December 1997. Similar
information was obtained retrospectively from the case records of
600 randomly chosen patients who underwent colonoscopy between 1986
and 1990.
| |
Colonoscopic procedures and surveillance |
Colonoscopies were performed on patients referred by a general
practitioner or specialist either for surveillance or for
investigation of symptoms. Through concessions available until mid
1996, the service was equally available to all patients irrespective
of financial resources. Fibreoptic colonoscopes were used
before 1990, and video colonoscopes after then. Patients were
lightly sedated with fentanyl (100 µg) and midazolam (2.5-5
mg). Treatment (eg, polypectomy, CRC resection) was given as
necessary.
Surveillance recommendations were mostly included in procedure
reports and passed to both the patient and the referring doctor. While
these recommendations changed over time consistent with published
guidelines,3,7 a general summary
is: - Annual surveillance for either active ulcerative
pancolitis of seven years' duration or longer or a previous
malignancy plus a family history of hereditary non-polyposis
colorectal cancer (HNPCC);3,7
- One- to two-yearly surveillance for a previous CRC before 50 years
and for those older than 25-40 years with either CRC developing in a
first-degree relative before 50 years or a family history of HNPCC;
- Two- to five-yearly surveillance for longstanding quiescent
pancolitis or active limited colitis, CRC or polyps in a first-degree
relative, previous CRC, or large (>1 cm diameter) or multiple
colonic polyps; and
- Five- to 10-yearly surveillance, depending on age, for a small
adenomatous polyp.
| |
Colorectal cancer diagnoses |
All patients diagnosed with CRC in Townsville between January
1994 and December 1997 were identified retrospectively by
searching the computerised databases of all three pathology
services, three hospitals, three endoscopy services, three
colonoscopists (including myself) and one oncology service
provider in Townsville, and from the CRC audits maintained by the six
surgeons in Townsville. From 1995, patients diagnosed with CRC were
also identified prospectively by clinicians and institutions.
Patients diagnosed with CRC who had had a previous colonoscopy
performed by myself at the Mater Private Hospital were identified to
January 1998.
Clinical records of all patients diagnosed with CRC were audited by
myself. A modified Dukes classification (A, B, C or D) was used for
staging cancer spread.8,9 A malignant polyp was
classified separately if colonoscopic resection was regarded as the
definitive treatment. In cases of synchronous lesions, the lesion
with the most invasive grading was registered.
| |
Incidence of CRC |
Colonoscopy population: The incidence of CRC was
calculated as the number of cases per thousand patient-years for the
period 1994-1997 for patients who had had a previous colonoscopy and
still lived in the region in 1997 (colonoscopy population).
Residence was determined from the electoral register current in
January 1997,10 which is considered
reliable as voter registration is compulsory in Australia. To reduce
mismatch errors caused by individuals with identical names, only
patients with a known middle name (duplication rate, 0.3%) were
cross-referenced against voters who also had a recorded middle name
(duplication rate, 1.3%). The number of patients without a middle
name who were still resident was estimated and added to the above on the
assumption that the proportion still resident would be the same in the
groups with and without a known middle name.
CRC incidence in each year was calculated for patients who had
undergone previous colonoscopy up until the previous calendar year.
For example, the incidence of CRC in 1994 was calculated for patients
who had undergone previous colonoscopy up to 1993. Age of colonoscopy
patients was determined for the year of incidence. The number of
patient-years was the total for all patients in a given age range who
had previously had a colonoscopy up to 1993, 1994, 1995 and 1996.
Community: The incidence of CRC in the remaining
population (community) was determined from the number of cases that
occurred between 1994 and 1997 in people not registered as a
colonoscopy patient per the region's population less the
colonoscopy population. Population data were obtained from
the August 1996 census undertaken by the Australian Bureau of
Statistics.9
| |
Statistical analyses |
Binary data were compared in two by two contingency tables using
chi-squared analyses.12 The age-standardised
incidences of CRC in colonoscopy patients versus the community were
tested for the hypothesis that the ratios were equal to
one.13 |
| |
Results |
| |
Colonoscopies |
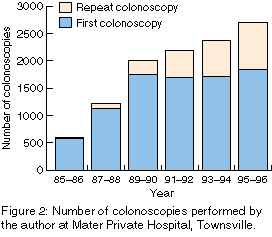
From mid 1985 to the end of 1996, I performed 11 148 colonoscopies on
8430 patients (Figure 2). The number
increased steadily, from 590 in the 18 months 1985-1986 to 2708 in the
two years 1995-1996. The number of repeat colonoscopies increased
from 12 (2.0%) in 1985-1986 to 875 (32.3%) in 1995-1996.
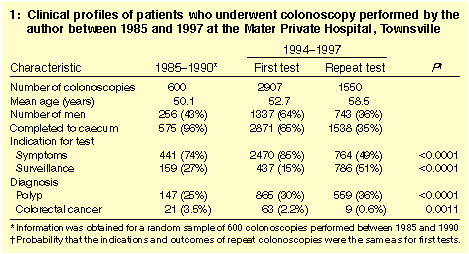
Clinical and procedural characteristics are summarised in Box 1.
Slightly more women than men had colonoscopies. Patients undergoing
repeat colonoscopies were an average six years older than those newly
referred and were more likely to have had surveillance for increased
CRC risk as the primary indication (50.7% of repeat colonoscopies
versus 15.0% of first colonoscopies; P < 0.0001). Primary
indication also varied with time. For example, an abnormal barium
enema was a common indication before 1991 (7.0%), but accounted for
few after 1994 (0.3%; P < 0.0001). In contrast, a family
history of polyps or CRC accounted for a greater proportion of
colonoscopies after 1994 (10.6% of first and 11.2% of repeat
colonoscopies) than before 1991 (6.3%; P < 0.0001).
From the outset, the caecal completion rate exceeded 95%, and from
1994 it was 98.9% overall and 99.5% in those without a malignant
obstruction. Polyps were diagnosed (and removed) in a greater
proportion of repeat than first colonoscopies (36.1% versus 29.8%;
P < 0.0001). Both these rates were higher than for
colonoscopies performed before 1991 (24.5%; P < 0.0001).
However, CRC was diagnosed less often in repeat than in first
colonoscopies (0.6% versus 2.2%; P = 0.001).
| |
Resident populations |
Complete details, including a middle name, were recorded for 5762 of
the 8430 colonoscopy patients (68.4%), and 4200 of these (72.9%) were
registered voters and residents of the Townsville region in 1997. A
surname and one given name only were recorded for the remaining 2668
patients -- 1913 from the hospital's computer register and 755 from
the work ledger. This gave an estimated total number of resident
colonoscopy patients of 6195 in 1997.
| |
Colorectal cancers |
Between 1994 and 1997, 476 new CRCs were diagnosed in residents of the
Townsville region, with 474 in people aged over 35 years. Eighteen
were in patients who had had a previous colonoscopy; each of these was
diagnosed per colonoscopy by myself, nine at the study hospital
(registered in the procedural database and shown in Box 1), and the
remainder elsewhere.
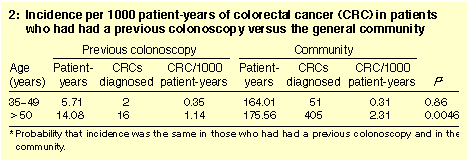
Incidences of CRC between 1994 and 1997 are shown in Box 2. In people
aged over 50 years, the annual incidence of CRC in the colonoscopy
population (1.14) was just less than half that in the remaining
community (2.31; P = 0.0046).
Between July 1985 and January 1998, I diagnosed 31 CRCs in 29 patients
who had had a previous colonoscopy (two patients had a second CRC
diagnosed two years after the first in each case). All but one of these
patients had a personal or family history that warranted
surveillance, and 21 had been enrolled in surveillance
programs, with 20 (65%) having had multiple previous colonoscopies
(mean, 3.4; range, 2-9). For 20 of the CRCs, planned surveillance was
the indication for the repeat colonoscopy. Among the 11 people with
symptoms as the primary indication, these symptoms were considered
unrelated to the CRC in at least three. The time between most recent
previous colonoscopy and diagnosis averaged 37 months (range, 3-136
months).
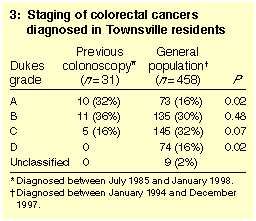
Staging of CRCs is shown in Box 3. Metastatic spread from CRCs was less
common in patients who had had a previous colonoscopy than in the
community; only five of the 31 cases in colonoscopy patients were
graded C, and none were graded D, while 219 of the 458 community cases
(48%) were graded C or D (P < 0.001). Among the five
colonoscopy patients with metastatic spread (Dukes C), the time
between most recent previous colonoscopy and diagnosis was 19, 24,
29, 32 and 70 months, respectively.
|
| |
Discussion |
The study evaluated a colonoscopic service that followed and
promoted contemporary surveillance guidelines similar to those
currently recommended by professional cancer and
gastroenterological societies.3 The incidence data suggest,
but do not prove, that colonoscopic surveillance confers a benefit.
Age-standardised incidence of CRC among people aged 50 years and over
in the Townsville region was lower among those who had had a previous
colonoscopy, along with any treatment considered necessary
(eg, polypectomy or bowel resection), than in the community. This was
despite the fact that many of those who had had a previous colonoscopy
had a personal or family history likely to increase their risk of
developing CRC. Furthermore, the CRCs that occurred in those who had
had a previous colonoscopy were of a lower Dukes grade than those
occurring in the community.
The difference in incidence suggests but does not prove that
colonoscopy is protective against CRC. CRC incidence for 1994-1997
in those who had had a previous colonoscopy may have been reduced, at
least partly, by detection of subclinical CRCs during their pre-1994
colonoscopies. On the other hand, selection bias suggests that these
people would develop more CRCs than the general population. The
impact of each of these factors could not be measured, and there is no
historical benchmark or matched population for comparison of
outcomes. However, the result does suggest that colonoscopy confers
a benefit, possibly because of removal of polyps and certainly
because of detection and treatment of subclinical CRCs.
In people aged 35-49 years, CRC incidence in those who had had a
previous colonoscopy was similar to that in the community. Without a
control group, a benefit of colonoscopy cannot be dismissed, as the
colonoscopy group was expected to have higher CRC incidence.
However, it is evident that, because of the large number of people aged
35 to 49 years and the low incidence of CRC, surveillance must be
targeted to be effective.
Polypectomy rate was high, and higher in repeat than in first
colonoscopies. This also implies that patients having repeat
colonoscopies had increased risk of developing CRC.14 While the high
polyp rate may have been due to their older average age,15 the latter
would also be expected to increase the CRC rate, which did not occur.
Given an expectation that all lesions seen at the previous
colonoscopy had been dealt with, this outcome (high polyp versus low
CRC rate) validates the selection criteria for surveillance. The
higher polypectomy rate after 1994 compared with that before 1990
probably relates to other circumstances, such as a higher caecal
completion rate, while both indices probably reflect improved
instrument technology.
Thirty-one new primary cancers developed in 29 colonoscopy
patients, with two-thirds diagnosed by planned surveillance
colonoscopy. Metastatic spread occurred in only five of these
patients. These findings confirm, firstly, that new CRCs will
develop and, secondly, that outcome can be improved through early
(subclinical) diagnosis.3,16
The number of cancers diagnosed in patients who had had a previous
colonoscopy was of concern and suggested lesions might have been
missed in the earlier examination. Colonoscopy, even when performed
by an expert, does not identify all small lesions, while adverse
conditions sometimes obscure gross pathology.17 Colon
morphology, quality of the bowel preparation, instrument
capabilities and operator proficiency may also impose
limitations.3,18 However, substandard
colonoscopy is unlikely to have been responsible. The caecum was
reached at a rate exceeding the accepted standard (95%),3 and CRCs were
observed in all parts of the bowel, arguing against an
operator-dependent blind spot.
Possibly, the comparatively large number of CRCs found in people
undergoing colonoscopic surveillance was simply the outcome of
increasing enlistment of an appropriate, at-risk cohort. Although
most sporadic CRCs evolve slowly through malignant transition in a
polyp, this sequence is truncated or absent for some sporadic CRCs and
for CRCs developing in patients with ulcerative colitis or a genetic
predisposition.19 It is unrealistic to
imagine that surveillance colonoscopy with polypectomy as
necessary will much reduce CRC incidence in such at-risk
populations. Indeed, it might conceivably increase apparent
incidence by uncovering subclinical CRCs.
The results support the current practice of targeting individuals
with recognised risk factors for surveillance colonoscopy.
However, it is important to explain to patients that surveillance
does not provide complete protection and that new CRCs are
inevitable. Early diagnosis through repeated testing is the
essential component of surveillance-derived protection.
|
| |
References |
- Parkin DM, Pisani P, Ferlay J. Estimates of the world-wide
incidence of eighteen major cancers in 1985. Int J Cancer
1993; 54: 594-606.
-
SEER Program (National Cancer Institute). Surveillance,
epidemiology, and end results (SEER) program. Bethesda, Md:
National Cancer Institute, 1973-1992.
-
Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer
screening: clinical guidelines and rationale.
Gastroenterology 1997; 112: 594-642.
-
Bolin TD, Korman MG. How can we reduce the incidence and mortality of
colorectal cancer [editorial]? Med J Aust 1997; 166:
175-176.
-
Macrae FA. Screening for colorectal cancer, 1996 [editorial].
Med J Aust 1996; 165: 102-105.
-
Bell JC, McCredie M, Coates MS, Armstrong BK. Trends in colorectal
cancer incidence and mortality in New South Wales, 1973-1992. Med
J Aust 1997; 166: 178-181.
-
Mecklin J-P, Jarvinen HJ, Peltokallio P. Cancer family syndrome.
Genetic analysis of 22 Finnish kindreds. Gastroenterology
1986; 90: 328-333.
-
Astler VB, Coller FA. The prognostic significance of direct
extension of carcinoma of the colon and rectum. Ann Surg 1954;
139: 846-851.
-
Dunlop MG. Polyps and carcinoma. In: Shearman DJC, Finlayson N,
Camillieri, Carter D, editors. Diseases of the gastrointestinal
tract and liver. 3rd ed. New York: Churchill Livingstone, 1997:
1399-1448.
-
Australian Electoral Commission. Elector information access
system. Electoral roll information for Queensland. Canberra:
Australian Electoral Commission, 1997.
-
Australian Bureau of Statistics. 1996 census of population and
housing. Community profile, Canberra: ABS, 1996 (Cat. no. 2020.0).
-
Approximate significance for contingency tables. In: Matthews
DE, Farewell VT. Using and understanding medical statistics. 2nd ed.
Basel: Karger, 1988: 20-66.
-
The binomial distribution. In: Snedecor GW, Cochran WG.
Statistical methods. 8th ed. Ames: Iowa State University Press,
1989: 107-134.
-
Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer
after excision of rectosigmoid adenomas. N Engl J Med 1992;
326: 658-662.
-
Williams AR, Balasooriya BAW, Day DW. Polyps and cancer of the
large bowel: a necropsy study in Liverpool. Gut 1982; 123:
835-842.
-
Mandel JS, Bond JH, Church TR, et al. Reducing mortality from
colorectal cancer by screening for fecal occult blood. Minnesota
Colon Cancer Control Study. N Engl J Med 1993; 328: 1365-1371.
(Published erratum appears in N Engl J Med 1993; 329: 672.)
-
Rex RK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of
adenomas determined by back-to-back colonoscopies.
Gastroenterology 1997; 112: 24-28.
-
Baille J, Ravich WJ. On endoscopic training and procedural
competence. Ann Intern Med 1993; 118: 73-74.
-
Kuramoto S, Oohara T. Flat early cancers of the large intestine.
Cancer 1989; 64: 950-955.
(Received 1 May, accepted 21 Dec, 1998)
|
| | Author's Details |
42 Ross River Road, Townsville, QLD.
John Croese, MD, FRACP, Gastroenterologist.
Reprints will not be available from the author.
Correspondence: Dr J Croese, 42 Ross River Road, Townsville, QLD 4812.
Email: jcroeseATmedeserv.com.au
| |
| |
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
|
| |
Back to text
Back to text
Back to text
Back to text
Back to text
|
|









