Eosinophilic oesophagitis is a chronic inflammatory disease characterised by eosinophilic inflammation of the oesophagus and sometimes scarring, and is associated with difficulty swallowing, dyspepsia and choking. Since spontaneous resolution is rare, therapeutic options have centred around use of medication or dietary manipulation. First described in the mid‐1990s, the condition is now recognised to affect about 42 per 100 000 of the adult population and 34 per 100 000 of the paediatric population.1 These data may be an underestimate of the true prevalence of eosinophilic oesophagitis due to the diagnosis requiring oesophageal biopsies and a high index of suspicion. Up to 12–23% of patients undergoing endoscopy for dysphagia and 50% of those presenting with food bolus obstruction have eosinophilic oesophagitis as the cause of their symptoms.2,3 The prevalence of eosinophilic oesophagitis has steadily (and in some populations exponentially) increased, and although prevalence data from Australia are lacking, anecdotally a similar phenomenon has been observed.2,4
Presentation and diagnosis
Eosinophilic oesophagitis is associated with symptoms of dysphagia, odynophagia, dyspepsia, chest pain, choking and food avoidance. Generalised symptoms are observed, particularly in paediatric patients, such as nausea, abdominal pain, food refusal, slow eating or compensation by drinking liquids and subsequent failure to thrive.5 Mimicking conditions of eosinophilic oesophagitis can include oesophageal dysmotility, anxiety or functional dyspepsia. If the diagnosis is suspected, gastroenterology referral for endoscopy and oesophageal biopsies are appropriate to examine for eosinophilic infiltrate. The diagnosis, defined by the Appraisal of Guidelines for Research and Evaluation (AGREE) Consortium, includes meeting the following criteria: i) symptoms of oesophageal dysfunction; ii) confirmation of histological inflammation with ≥ 15 eosinophils per high power field (or ~ 60 eosinophils per mm2) on oesophageal biopsy; and iii) exclusion of non‐eosinophilic oesophagitis disorders that could cause secondary oesophageal eosinophilia.6 Routine IgE mediated allergy testing has not been considered useful.7 Around 10% of cases of eosinophilic oesophagitis are genetic2 and screening of parents or siblings is indicated.
Medical therapy
The treatment goals are threefold: i) to control oesophageal eosinophilia (with a goal to achieve < 5 eosinophils per high power field), which leads to ii) improvement in symptoms of oesophageal dysfunction and iii) the prevention and/or reversal of fibrostenotic complications.
Proton pump inhibitors and topical steroids are the most widely used medical treatments with 50% and 65% remission rates respectively; however, the landscape is rapidly changing and there are a number of novel agents under development which show promise, including monoclonal antibodies targeting inflammatory cytokines.8,9,10 Symptom absence or improvement alone does not always correlate with endoscopic changes and, therefore, given the risks of scarring, treatment should be guided by eosinophilia counts on repeat endoscopies. Nevertheless, many patients do not want to take medications and, as eosinophilic oesophagitis is in essence an allergic disorder, avoidance of antigen triggers via dietary restriction is attractive.
Dietary therapy
Like medical therapy, dietary therapy for eosinophilic oesophagitis has evolved. Initial interventions in paediatric populations using solely elemental nutritional formula demonstrated up to 100% efficacy.11 This formula, which was comprised of individual amino acids, fatty acids, and sugars, allowed avoidance of suspected dietary antigens. Remission rates in observational trials were high, but the poor palatability of the formula was a major limitation, especially in adults.
Given the limitations of the elemental diet, the six‐food elimination diet (6FED) was developed. A retrospective observational study defined the exclusion foods based on common dietary allergens in children,12 and another study investigated positive skin prick and patch testing in children with eosinophilic oesophagitis.13 The 6FED requires the exclusion of animal/cow's milk proteins, wheat, eggs, soy, seafood and nuts.12 Given the restrictive nature of this diet, less restrictive forms, such as the four‐ and two‐food elimination diets (4FED and 2FED), have been proposed, with improved tolerance but at the cost of decreased efficacy (Box 1).
The efficacy of the 6FED has been examined in two systematic reviews, with remission rates reported as 61.3% (95% confidence interval [CI], 53.0–69.3%) and 72.1% (95% CI, 65.8–78.1%).7,11 There is substantial variability in remission rates reported across studies (Box 1), likely due to the heterogeneity in remission/response definition, study design, paediatric versus adult cohorts, adherence to diet, and duration of intervention. Furthermore, studies rarely report compliance to dietary therapy, nutritional implications of a restricted diet or details on dietary education delivery, which is important given that appropriate dietary education by a specialist dietitian has demonstrated benefit in other conditions that require lifelong dietary manipulation, such as coeliac disease (Box 2).
In clinical practice in Australia, dietitians educate patients on the strictest approach, such as avoiding gluten in coeliac disease, as it is unknown if tolerance may change over time to dietary triggers. Unfortunately, compared with less restrictive elimination diets, the 6FED has been shown to be more expensive, less accessible for patients to follow, reduces patient's health‐related quality of life (while improving it in others), increases weight loss, and has poor adherence, with only 42% of patients following their recommended dietary restrictions at 12 months.14,19,20,21 Another challenge of the 6FED is the requirement of an endoscopy after each food is reintroduced back into the diet, making it a difficult intervention in the Australian public hospital system, where access to endoscopy is limited. Hence, there is increasing interest in less aggressive approaches that minimise health care ultilisation and cost.
To address these concerns, a step‐up method of a 2FED (cow's milk and/or all animal milk, wheat and/or gluten) or a 4FED (cow's milk and/or all animal milk, wheat and/or gluten, eggs, soy and/or legumes/peanuts) has been proposed, with remission rates of 43% and 54% respectively when including drop‐outs.14 These diets are less efficacious, but fewer endoscopies are required to identify the common triggers, which in 70% of cases is animal milk, followed by wheat (48%) and eggs (27%).7 Importantly, 70% of patients responding to a 2FED have a single food trigger (animal milk or gluten‐containing cereals) and, thus, patients taking a 6FED approach may be on an overly restrictive diet.14
Recently, the first multicentre, randomised, open label trial comparing a one‐food elimination diet (1FED; animal milk) with 6FED (animal milk, wheat, egg, soy, fish and shellfish, and peanut and tree nuts) for six weeks has been published with surprising results.19 One‐hundred and twenty‐nine adults with active symptomatic eosinophilic oesophagitis were randomly allocated to either a 6FED (n = 62) or a 1FED (n = 67). Dietary education was delivered by trained dietitians, with high adherence rates (97%), according to food diaries and questionaries, and with minimal drop‐outs. On intention‐to‐treat analysis there was no difference in histological remission rates for the 6FED compared with the 1FED (25/62 [40%] v 23/67 [34%] respectively; P = 0.58). This result was unexpected because previous observational data suggest a 70% remission rate with the 6FED. The authors hypothesised their study had a reduced selection bias, with intention‐to‐treat analysis being used rather than per protocol, education was delivered in a comprehensive way and represents real‐world data. Notably, unintentional weight loss was significantly higher in the 6FED compared with the 1FED (‐2.2 kg [standard deviation (SD), 2.5] v ‐1.1 kg [SD, 2.6]; P = 0.027); however, no other nutritional measures were examined, such as disordered eating behaviours or fear of foods. This study is crucial in exploring the level of restriction required in eosinophilic oesophagitis, as dietary restrictions are often prescribed without consideration for their potential harm and with poor understanding of their mechanism. High quality clinical trials to evaluate therapeutic diets in the same way as medical therapy are critical to observe not only efficacy but also adherence and safety.
Dietary mechanism
Eosinophilic oesophagitis is hypothesised to be driven by an impaired epithelial barrier function, resulting in type 2 helper cell inflammatory response. There is a response to removal of dietary triggers with the reduced expression of interleukin‐4 in CD4+ CD154+ T cells and total reduction of these in responders to an elimination diet, yet mechanisms of how individuals become sensitised to these food allergens is still being investigated.19 It has been suggested that aeroallergens may also be implicated in triggering eosinophilic oesophagitis in a subset of patients.22 Current practice is to restrict the suspected food allergen completely from the diet, in the same way as gluten is avoided in coeliac disease. However, we have no data on the level of restriction required (such as whether there is a threshold for tolerance), which proteins in the foods are driving the allergic response (eg, gluten v α‐amylase/trypsin inhibitors), and whether tolerance changes over time as is the case with other IgE‐related food allergies. This is relevant because there is variability in the types of foods and, therefore, proteins that are restricted in observational and interventional studies. It is frustrating that formal allergy testing (skin prick or patch testing) is also not particularly helpful for predicting response to dietary antigens. Twelve studies have used this approach with combined remission rates of 45.7% (95% CI, 32.0–59.7%), which is lower than an empirical 1FED response of 51.4% (95% CI, 42.6–60.1%).7 To refine this approach, more work is needed to understand the mechanism of dietary allergens to improve our understanding and treatment of the disease, given that dietary proteins are hypothesised to be the drivers of eosinophilic infiltration into the oesophagus in susceptible individuals.
Clinical challenges
Although dietary therapy appears equally as effective as medical therapies, it is a somewhat blunt and highly restrictive treatment with little high quality data available. Poorly implemented dietary therapy runs the risk of inadvertent antigen exposure (and failure to respond) or, conversely, over‐restriction, with risks of malnutrition and disordered eating and, thus, a multidisciplinary team inclusive of a specialised dietitian is required. Challenges with implementing dietary therapy also include difficulties of evaluating outcomes. Unfortunately, as symptoms correlate poorly with histological inflammation, repeat endoscopy and biopsy are required to assess response to dietary exclusions and/or reintroductions,8,23 and the optimal duration of dietary exclusion is not well defined. Despite most studies using six weeks, there are data that suggest that patients who have not met the histological criteria for remission but have an improvement in symptoms and/or endoscopic appearance may continue to improve, with longer exclusion risking “wasted” endoscopies.24 This adds a significant clinical burden to already overstretched endoscopy waiting lists, particularly in the public system. Furthermore, endoscopy delays may lead to patients following a highly restrictive diet for longer than would otherwise be recommended, with the associated nutritional risk.25 There are emerging techniques (ie, clinic‐based nasal endoscopy or cytosponge) that seek to overcome these hurdles, but these are not yet available in clinical practice.26,27
Given these variable data, there is a need for multidisciplinary eosinophilic oesophagitis models of care in Australia, with dietitians imbedded to enhance research capabilities to bridge these evidence gaps and help to determine predictors of response, long term follow‐up, nutritional and patient‐reported outcomes of dietary restriction, and required level of dietary restriction to induce and maintain remission, and evaluate new bedside techniques in eosinophilic oesophagitis.
Box 1 – Efficacy of elimination diets in eosinophilic oesophagitis (EoE)
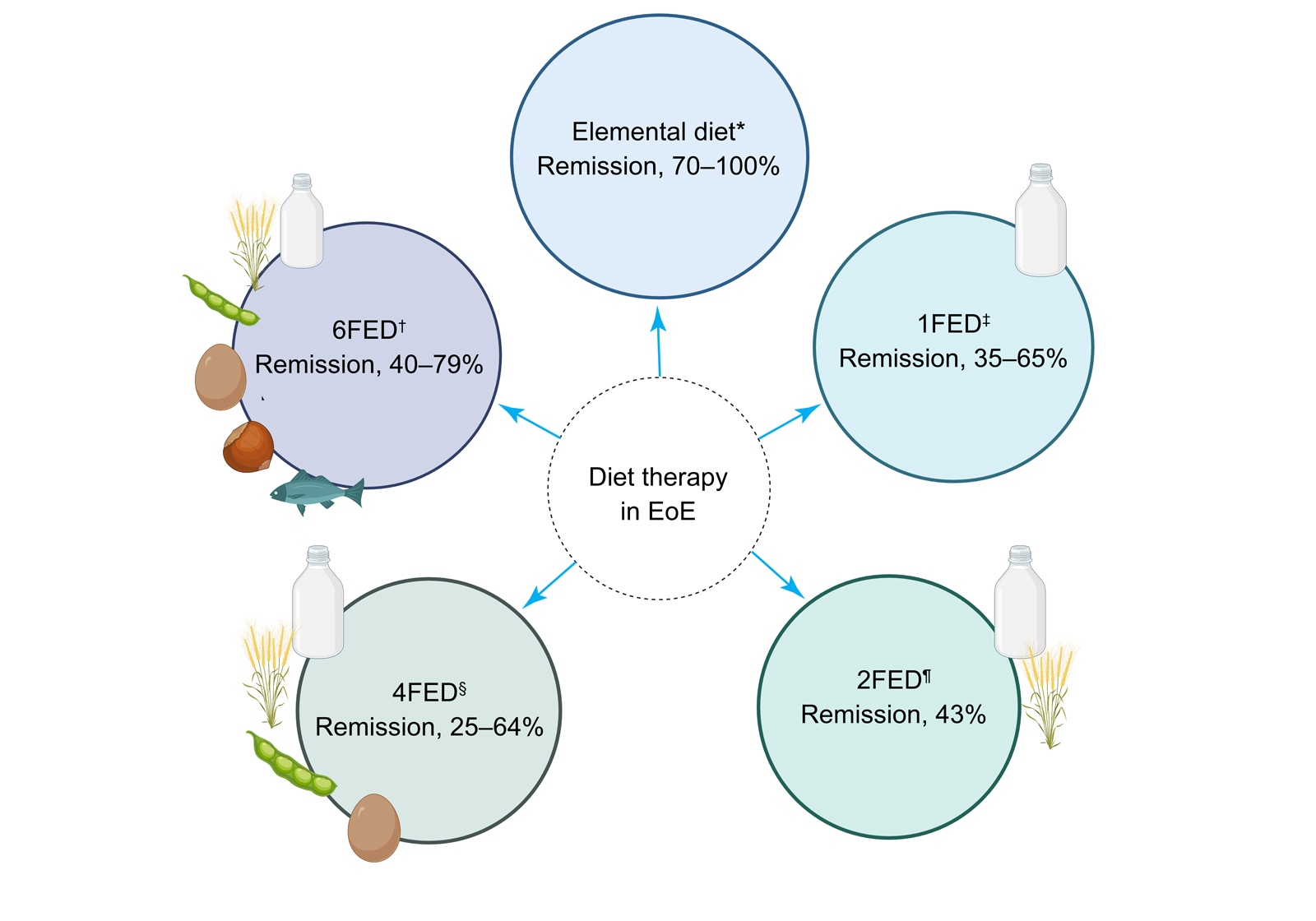
1FED = one‐food elimination diet; 2FED = two‐food elimination diet; 4FED = four‐food elimination diet; 6FED = six‐food elimination diet. * Arias et al;11 Kagalwalla et al.12 † Mayerhofer et al;7 Kagalwalla et al;12 Molina‐Infante et al;14 Eckmann et al.15 ‡ Kagalwalla et al;12 Molina‐Infante et al;14 Wechsler et al;16 Teoh et al;17 Mayerhofer et al.7 § Mayerhofer et al;7 Kagalwalla et al;12 de Rooij et al.18 ¶ Mayerhofer et at;7 Molina‐Infante et al.14 Figure created with Biorender.com.
Box 2 – Characteristics of studies examining elimination diets for eosinophilic oesophagitis
|
|
|||||||||||||||
|
|
|||||||||||||||
|
Total number of studies |
7 |
11 |
6 |
2 |
6 |
||||||||||
|
Dietitian involved |
71% |
55% |
33% |
0% |
83% |
||||||||||
|
Education described |
43% |
27% |
17% |
0% |
50% |
||||||||||
|
Adherence reported |
86% |
27% |
17% |
50% |
33% |
||||||||||
|
Weight change reported |
86% |
36% |
33% |
50% |
33% |
||||||||||
|
Follow‐up 12 months or beyond |
14% |
18% |
0% |
0% |
0% |
||||||||||
|
|
|||||||||||||||
|
1FED = one‐food elimination diet; 2FED = two‐food elimination diet; 4FED = four‐food elimination diet; 6FED = six‐food elimination diet. |
|||||||||||||||
Provenance: Commissioned; externally peer reviewed.
- 1. Navarro P, Arias Á, Arias‐González L, et al. Systematic review with meta‐analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population‐based studies. Aliment Pharmacol Ther 2019; 49: 1116‐1125.
- 2. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018; 154: 319‐332.
- 3. Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol 2007; 41: 356‐361.
- 4. Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child 2006; 91: 1000‐1004.
- 5. Straumann A, Katzka DA. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology 2018; 154: 346‐359.
- 6. Dellon ES, Liacouras CA, Molina‐Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE Conference. Gastroenterology 2018; 155: 1022‐1033.
- 7. Mayerhofer C, Kavallar AM, Aldrian D, et al. Efficacy of elimination diets in eosinophilic esophagitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2023; 21: 2197‐2210.
- 8. Dhar A, Haboubi HN, Attwood SE, et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut 2022; 71: 1459‐1487.
- 9. Lucendo AJ, Arias Á, Molina‐Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2016; 14: 13‐22.
- 10. Rank MA, Sharaf RN, Furuta GT, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA Institute and the Joint Task Force on Allergy‐Immunology Practice Parameters. Gastroenterology 2020; 158: 1789‐1810.
- 11. Arias A, González‐Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta‐analysis. Gastroenterology 2014; 146: 1639‐1648.
- 12. Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six‐food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4: 1097‐1102.
- 13. Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol 2002; 109: 363‐368.
- 14. Molina‐Infante J, Arias Á, Alcedo J, et al. Step‐up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2‐4‐6 study. J Allergy Clin Immunol 2018; 141: 1365‐1372.
- 15. Eckmann JD, Ravi K, Katzka DA, et al. Efficacy of atopy patch testing in directed dietary therapy of eosinophilic esophagitis: a pilot study. Dig Dis Sci 2018; 63: 694‐702.
- 16. Wechsler JB, Schwartz S, Arva NC, et al. A single‐food milk elimination diet is effective for treatment of eosinophilic esophagitis in children. Clin Gastroenterol Hepatol 2022; 20: 1748‐1756.
- 17. Teoh T, Mill C, Chan E, et al. Liberalized versus strict cow's milk elimination for the treatment of children with eosinophilic esophagitis. J Can Assoc Gastroenterol 2019; 2: 81‐85.
- 18. de Rooij WE, Vlieg‐Boerstra B, Warners MJ, et al. Effect of amino acid‐based formula added to four‐food elimination in adult eosinophilic esophagitis patients: a randomized clinical trial. Neurogastroenterol Motil 2022; 34: e14291.
- 19. Kliewer KL, Gonsalves N, Dellon ES, et al. One‐food versus six‐food elimination diet therapy for the treatment of eosinophilic oesophagitis: a multicentre, randomised, open‐label trial. Lancet Gastroenterol Hepatol 2023; 8: 408‐421.
- 20. Sheedy K, Patel N, Porter J, Silva H. Cost and accessibility of empiric food elimination diets for treatment of eosinophilic oesophagitis. Nutr Diet 2022; 79: 238‐246.
- 21. Wang L, Mara KC, Ravi K, et al. Predictors of histologic response to dietary therapy in eosinophilic oesophagitis. Aliment Pharmacol Ther 2022; 56: 1444‐1452.
- 22. Egan M, Atkins D. What is the relationship between eosinophilic esophagitis (EoE) and aeroallergens? Implications for allergen immunotherapy. Curr Allergy Asthma Rep 2018; 18: 43.
- 23. Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150: 581‐590.
- 24. Philpott H, Dellon E. Histologic improvement after 6 weeks of dietary elimination for eosinophilic esophagitis may be insufficient to determine efficacy. Asia Pac Allergy 2018; 8: e20.
- 25. Chang JW, Kliewer K, Haller E, et al. Development of a practical guide to implement and monitor diet therapy for eosinophilic esophagitis. Clin Gastroenterol Hepatol 2023; 21: 1690‐1698.
- 26. Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and safety of the cytosponge for assessing histologic activity in eosinophilic esophagitis: a two‐center study. Am J Gastroenterol 2017; 112: 1538‐1544.
- 27. Philpott H, Nandurkar S, Royce SG, Gibson PR. Ultrathin unsedated transnasal gastroscopy in monitoring eosinophilic esophagitis. J Gastroenterol Hepatol 2016; 31: 590‐594.






Open access:
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
Jessica Fitzpatrick and Sarah Melton are supported by a Crohn's Colitis Australia PhD Scholarship.
Rebecca Burgell has received speaker fees from Falk Pharmaceutical. Jessica Fitzpatrick has received speaker fees from Pepsi Co.