Clinical record
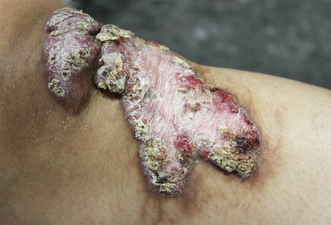
A 32‐year‐old woman presented with a painless but cosmetically bothersome chronic indolent lesion on her left knee. The lesion arose from a previous wound site that developed about four weeks following a fall 20 years prior in the coral reefs of New Zealand. She was systemically well and had no significant medical or family history. On examination, there was a large (about 9 × 5 cm in size), well defined pink plaque over the left patella with a hyperkeratotic, crusted and verrucous surface (Box 1). Surrounding the wound were areas of post‐inflammatory hyperpigmentation. There were no other skin lesions nor associated lymphadenopathy.
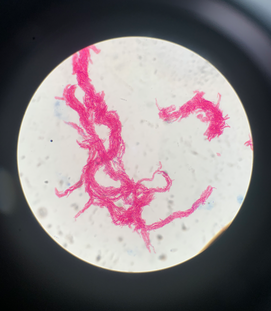
Initial differential diagnoses included keloid scarring, atypical mycobacterial or deep fungal infection, cutaneous malignancy, and granulomatous disease. A punch biopsy was subsequently performed. Histopathology showed epidermal hyperplasia, dermal inflammation consisting of lymphocytic infiltrates with a focal granulomatous component, and scarring within the deeper dermis (Box 2, A and B). There was no evidence of fungal organisms or acid‐fast bacilli on periodic acid‐Schiff and Ziehl–Neelsen staining respectively. After a five‐week incubation period, tissue culture with Ziehl–Neelsen stain confirmed the presence of M. marinum (Box 3). No treatment was administered pending culture results.
The diagnosis of cutaneous M. marinum infection was made after correlating the clinical history, histopathological findings, and culture results. After consultation with an infectious diseases specialist, the patient was commenced on doxycycline, clarithromycin and rifampicin for a minimum of six weeks before being lost to follow‐up. Furthermore, she was referred to plastic surgery for consideration of additional surgical resection due to the lesion's extent, but failed to attend her consultation.
Discussion
Mycobacterium marinum is a slow growing, non‐tuberculous, acid‐fast, non‐motile bacillus that thrives in temperate, hot and humid aquatic environments.1 Inoculation can occur through occupational and recreational exposure, typically following a traumatic injury in a contaminated salt‐ or freshwater environment.2,3 The association with fish tanks is classical, earning the disease the moniker “fish tank granuloma”.2 In a series of 63 cases from France, 84% were linked to fish tank exposure.3,4 In Australia, however, exposure to M. marinum is more likely to occur from outdoor activities. The clinical appearance is a solitary, red‐to‐violaceous papule, nodule or plaque on the extremities.3,4 The surface may be crusted or verrucous and may progress to ulceration or disseminated infection in severe cases.4 The infection may extend to deeper tissues, causing tenosynovitis, osteomyelitis or septic arthritis.1 Affected individuals may delay seeking treatment for several months, as infection can be indolent and painless.2,3 Lesions may also heal spontaneously.5
The diagnosis is made by culturing the organism from tissue biopsy. Growth occurs after a median incubation period of 21 days (range, 5–210 days) in Löwenstein–Jensen agar culture at 28–32°C.4 Polymerase chain reaction amplification techniques using Mycobacterium genus‐specific primers have emerged as a rapid diagnostic test, enabling identification of M. marinum directly from the tissue sample.1 On microscopy, M. marinum appears as a non‐motile pleomorphic rod with branching. It is photochromogenic, with colonies turning yellow to orange on exposure to light.3 Ziehl–Neelsen stain will show acid‐fast bacilli, but yield can be difficult as the number of mycobacteria in clinical specimens is low.2,6 It is important to culture biopsies to allow for differentiation from other non‐tuberculous mycobacterial infections (Mycobacterium avium‐intracellulare, Mycobacterium abscessus, Mycobacterium fortuitum, Mycobacterium chelonae) which can also present as chronic non‐healing wounds.7
There is no standard treatment for M. marinum infection. Rifampicin is the most effective drug reported based on MIC90 (concentration that inhibited growth of 90% of isolates) below 0.5 mg/mL.4 Mild infections limited to the skin and soft tissue have been treated effectively with tetracyclines (minocycline and doxycycline). Clarithromycin has also been used successfully both as monotherapy for superficial lesions and as part of a multidrug regimen for deeper infections.1,4,5 Other drugs with reported efficacy include ethambutol, trimethoprim/sulfamethoxazole, imipenem, linezolid, ciprofloxacin and moxifloxacin.5 Amikacin has been reported as an effective addition to a multidrug regimen in refractory cases.4,5
Input from an infectious diseases specialist should be sought. The number of agents and the duration of therapy are often proportionate to the burden of disease, clinical response and immune status.5 Single drug therapy may be effective for mild superficial disease, with multidrug therapy (with or without surgical debridement) favoured for extensive disease.5 Treatment duration should then be tailored to response, noting that the rate of clinical response is variable; at least four to six weeks of therapy should be administered before inadequate response should be suspected.4,5 Surgical treatment allows for source control of infection, preservation of limb function and better cosmetic outcomes.5 M. marinum infection is very rare; in a series of 260 aquatic wounds in tropical Australia, there was not a single case of M. marinum identified.8 Although rare, one should consider M. marinum infection in chronic indolent lesions with a history of trauma in an aquatic environment, as in our case.
Lessons from practice
- Mycobacterium marinum lives in aquatic environments, particularly salt water, aquariums and pools; infection occurs when cut or abraded skin is exposed to a contaminated source.
- Clinical suspicion is necessary for the diagnosis of M. marinum infection; a chronic indolent lesion with a history of trauma to the limb in an aquatic environment, due to occupational or recreational activities, is a salient feature of the patient's history.
- Diagnosis is made by culturing the organism from tissue biopsy in a Lowenstein–Jensen agar medium incubated at 28–32°C; communication with the pathologist is important to ensure that appropriate culture conditions and observation periods are in place to assist with diagnosis.
- Consultation with an infectious diseases specialist is encouraged since treatment can be challenging and often requires multidrug regimens for extended periods of time; surgical debridement and resection of the lesion may be indicated in cases refractory to treatment with antibiotics.
Box 1 – A well defined hyperkeratotic growth over the left knee with an eroded, crusted and verrucous surface
Box 2 – Histopathological examination shows epidermal hyperplasia and dermal inflammation consisting of mainly lymphocytic infiltrates with a focal granulomatous component*
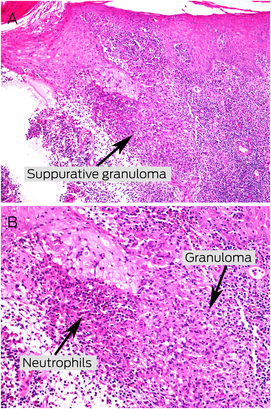
* A few neutrophils, eosinophils, and plasma cells are also seen. There is scarring within the deeper dermis (A: haematoxylin and eosin stain, × 200; B: haematoxylin and eosin stain, × 400).
Provenance: Not commissioned; externally peer reviewed.
- 1. Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis 2015; 21: 7.
- 2. Aubry A, Mougari F, Reibel F, Cambau E. Mycobacterium marinum. Microbiol Spectr 2017; 5: https://doi.org/10.1128/microbiolspec.TNMI7‐0038‐2016.
- 3. Ang P, Rattana‐Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol 2000; 39: 343‐347.
- 4. Aubry A, Chosidow O, Caumes E, et al. Sixty‐three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med 2002; 162: 1746‐1752.
- 5. Rallis E, Koumantaki‐Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother 2007; 8: 2965‐2978.
- 6. Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis 2000; 31: 439‐443.
- 7. Pennington KM, Vu A, Challener D, et al. Approach to the diagnosis and treatment of non‐tuberculous mycobacterial disease. J Clin Tuberc Other Mycobact Dis 2021; 24: 100244.
- 8. Vardanega J, Smith LK, Smith S, et al. Animal bite wounds and their management in tropical Australia. Int J Infect Dis 2022; 118: 1‐9.






Patient consent
The patient gave written consent for publication.
Open access:
Open access publishing facilitated by The University of Queensland, as part of the Wiley – The University of Queensland agreement via the Council of Australian University Librarians.
We thank Louis Pool, Dermatopathologist at Sullivan Nicolaides Pathology, for provision of the histopathology images and commentary, and Meryta May, Paediatric Infectious Disease Specialist and Microbiologist at Sullivan Nicolaides Pathology, for provision of the microscopy images and commentary used in this article.
No relevant disclosures.