The known: Primary aldosteronism, indicated by an elevated plasma aldosterone‐to‐renin ratio (ARR), is the most frequent endocrine cause of hypertension. Little is known about the relationship between ARR and blood pressure in Indigenous Australians, among whom the prevalence of hypertension is high.
The new: At the age of 32–35 years, screening ARR values exceeded 70 pmol/mU for 26% of urban Indigenous participants and 28% of non‐Indigenous participants. Higher ARR was associated with higher systolic blood pressure only in the non‐Indigenous group.
The implications: Targeted testing for primary aldosteronism is warranted as the first step to better quantifying its prevalence among Indigenous people in the Northern Territory.
Rates of hypertension and cardiovascular disease are higher among Indigenous than non‐Indigenous Australians in all age groups.1,2 The likelihood of hypertension is at least 30% greater for Indigenous people in all age groups;2 one long term study found that the incidence of hypertension among Indigenous adults was almost twice that for the general Australian population.3 Reducing the prevalence of cardiovascular disease is critical for reducing the life expectancy gap between Indigenous and non‐Indigenous Australians.4
Treating potentially curable causes of hypertension is important for effective management. Primary aldosteronism, the most frequent endocrine cause of hypertension, is underdiagnosed. It should be identified as early as possible because of its consequences for cardiovascular disease, including hypertension, myocardial infarction, arrhythmia, cardiac failure, and stroke.5,6 It can be cured by adrenal surgery or effectively managed with medications. Primary aldosteronism is detected by measuring aldosterone and renin concentrations and calculating the aldosterone‐to‐renin ratio (ARR); an elevated or normal aldosterone level and low or suppressed renin concentration leads to an elevated ARR value, which suggests primary aldosteronism and should prompt further investigation.
The combination of higher serum aldosterone levels with suppressed renin concentration is associated with the development of hypertension in normotensive people aged 45–58 years.7 In a multinational study of 663 normotensive or mildly hypertensive people aged 30–60 years, suppressed or low renin concentration was associated with autonomous aldosterone production and impaired vascular function.8 Lower aldosterone levels were associated with better cardiovascular health at the population level in the United States Framingham Offspring study (mean age, 58 years).9 Taken together, these findings suggest that autonomous, renin‐independent aldosterone production is an important contributor to hypertension and vascular dysfunction.
Aldosterone and renin profiles for Indigenous Australians have not been reported, despite hyperaldosteronism being a modifiable risk factor for cardiovascular disease. The aims of our study were to evaluate aldosterone and renin levels, their association with blood pressure, and the prevalence of positive screening test results for primary aldosteronism among young Indigenous and non‐Indigenous adults in the Northern Territory.
Methods
The participants in our cross‐sectional study were members of the Aboriginal Birth Cohort (ABC) and the Top End Cohort (TEC) aged 32–35 years. The ABC includes 686 people born to Indigenous mothers at the Royal Darwin Hospital during 1987–1990; the TEC includes 192 people born to non‐Indigenous mothers in Darwin, recruited during 2007–2009. The recruitment and follow‐up of the two cohorts have been described elsewhere.10,11,12
Data collection
We collected sex, age, alcohol use, smoking status, height, weight, and waist circumference data for each participant.10 Body mass index (BMI) values were calculated and grouped as < 25, 25.0–29.9, or ≥ 30 kg/m2. Blood pressure was assessed and blood samples collected during 1 April 2019 – 31 December 2021. Blood pressure was measured three times (right arm) while the participant was seated after resting for 5–10 minutes, and the mean value recorded; grade 1 hypertension was defined by systolic blood pressure greater than 130 mmHg or diastolic blood pressure greater than 80 mmHg, grade 2 hypertension as systolic blood pressure greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg. ABC samples were grouped by residential location (urban or remote) because of the implications for sample handling.
Blood collection and analysis
Blood samples were collected before noon in community spaces (eg, community health centres, community halls, council spaces, school rooms) while the participant was seated. Serum and plasma were separated within two hours of collection and immediately frozen (–20°C). TEC and urban ABC samples remained frozen during storage and transfer to laboratories; remote ABC samples were transported on ice for up to two hours before serum and plasma separation.
Plasma aldosterone and direct renin concentrations were measured by Monash Pathology (Melbourne) using validated high throughput assays (DiaSorin LIAISON chemiluminescent immunoassays) and the Liaison XL analyser (DiaSorin). Plasma aldosterone concentration was reported in pmol/L; the between‐run analytical coefficients of variation were 9.5% at 188 pmol/L and 5.6% at 798 pmol/L. Direct renin concentration was reported in mU/L; the between‐run analytic coefficients of variation were 10.0% at 5.1 mU/L and 4.3% at 82.4 mU/L. The ARR was calculated by dividing plasma aldosterone concentration by direct renin concentration; an ARR exceeding 70 pmol/mU was deemed a positive screening test result for primary aldosteronism.13
Statistical analysis
Participant characteristics are reported as means with standard deviations (SDs), medians with interquartile ranges (IQRs), or numbers and proportions, as appropriate. The statistical significance of between‐group differences was assessed in Pearson χ2 or Fisher exact tests (categorical variables), linear regression (comparisons of means), or distribution comparisons (when medians are reported). Relationships between blood pressure and age, sex, waist circumference, BMI, plasma aldosterone concentration, direct renin concentration, and ARR were assessed using linear regression, both unadjusted and adjusted for other factors; we report beta values (estimated change per unit change in the predictor variable) with 95% confidence intervals (CIs). Analyses were performed in R 2022.07.2 (RStudio, Posit).
Ethics approval
The study was approved by the human research ethics committee of the Northern Territory Department of Health and Community Services and the Menzies School of Health Research (HREC 2018‐3257), including the Aboriginal Ethics Sub‐Committee (HREC 2018‐3258). Each cohort study participant provided written informed consent.
Results
A total of 255 ABC members (37% of the ABC cohort; 205 in remote, 50 in urban locations) and 76 TEC members (40% of the cohort) participated in the study. Mean BMI was 30.1 kg/m2 (SD, 5.4 kg/m2) for urban ABC participants, 26.5 kg/m2 for TEC participants (SD, 5.4 kg/m2), and 24.1 kg/m2 for remote ABC participants (SD, 5.9 kg/m2). Mean systolic blood pressure was 121 mmHg (SD, 16 mmHg) in the urban ABC group, 115 mmHg (SD, 13 mmHg) in the remote ABC group, and 117 mmHg (SD, 13 mmHg) in the TEC group; the differences in diastolic blood pressure were similar. The proportions of participants with grade 1 (TEC, 28%; ABC [urban], 36%; ABC [remote], 25%) or grade 2 hypertension (TEC, 7%; ABC [urban], 10%; ABC [remote], 5%) were similar in the three groups (Box 1).
Aldosterone, renin, and aldosterone‐to‐renin ratio
The median plasma aldosterone concentration was similar for all three groups. The direct renin concentration was 7.5 mU/L (IQR, 4.1–12.4 mU/L) in the TEC group, 12.4 mU/L (IQR, 5.1–19 mU/L) in the urban ABC group, and 29.3 mU/L (IQR, 15.0–52.9 mU/L) in the remote ABC group. The median ARR was 10 pmol/mU (IQR, 6–19 pmol/mU) in the remote ABC group, 28 pmol/mU (IQR, 16–70 pmol/mU) in the urban ABC group, and 43 pmol/mU (IQR, 26–74 pmol/mU) in the TEC group (Box 1, Box 2).
Thirteen urban ABC (26%) and twenty‐one TEC participants (28%) had ARRs exceeding 70 pmol/mU, as did six people in the remote ABC group (3%). Some test centres deem an ARR value greater than 50 pmol/mU as a positive screening test result;14 an ARR beyond this threshold was identified in eleven remote ABC participants (5%), sixteen urban ABC participants (32%), and 32 TEC participants (42%) (Box 1). Other test centres define a positive screening result as an elevated ARR (> 70 pmol/mU) together with plasma aldosterone concentration greater than 270 pmol/L;13 these criteria were met by three remote ABC participants (1%), eleven urban ABC participants (22%), and fifteen TEC participants (20%).
All three women in the remote ABC group who had elevated ARRs were using a progestogen contraceptive implant (etonogestrel); two of seven women in the TEC group with elevated ARRs and one urban ABC participant were using an oral contraceptive; a second urban ABC participant was using depot medroxyprogesterone (Depo‐Provera).
Aldosterone‐to‐renin ratio and blood pressure
Mean systolic and diastolic blood pressure values in each of the three groups were similar for participants with ARRs of 70 pmol/mU or less and for participants with ARRs that exceeded 70 pmol/mU. However, the numbers of participants with ARRs greater than 70 pmol/mU were small for the urban ABC (thirteen people) and rural ABC groups (six people). Seventeen of the twenty‐one TEC participants with ARRs above 70 pmol/mU were women (81%), and 31 of 55 of those with values below 70 pmol/mU (56%) (Box 3).
Systolic and diastolic blood pressure
After adjusting for age and BMI, mean systolic and diastolic blood pressure were lower for women than men in all participant groups; after adjusting for age and sex, greater waist circumference and BMI were associated with higher systolic and diastolic blood pressure in all participant groups. In the TEC group, higher ARR was associated with higher systolic blood pressure, after adjusting for age, sex, and BMI, but not in the two ABC groups. Neither plasma aldosterone concentration nor direct renin concentration alone were associated with changes in blood pressure in any participant group (Box 4).
Discussion
In this study, the median aldosterone level was similar in all three groups, but the median renin concentration was lower and the median ARR correspondingly higher in the TEC and urban ABC groups than in the remote ABC group. ARR values exceeded 70 pmol/mU in about one‐quarter of TEC and urban ABC participants, but in only six people in the remote ABC group (3%). The proportion of positive primary aldosteronism screening test results would be higher were lower ARR thresholds applied (30 or 50 pmol/mU13,14,15). Systolic and diastolic blood pressure levels were higher for men, and increased with waist circumference and BMI in all three study groups; systolic blood pressure increased with ARR in the TEC but not the ABC group.
Median renin concentration was higher in the remote ABC group than in the urban ABC and TEC groups despite similar median aldosterone concentrations. This finding may be related to blood samples being transported on ice from remote centres to a central laboratory; cryoactivation at temperatures of 0–4°C leads to higher measured renin levels.16,17 Lower salt consumption can also increase renin concentration,18 but similar median aldosterone values for all three groups suggest that differences in salt consumption did not influence renin levels. Consequently, we may have underestimated the prevalence of positive screening results among people in the remote ABC group.
The proportions of people with elevated ARR values were similar in the urban ABC (26%) and TEC groups (28%). The proportions were similar to that reported in a recent Australian prevalence study, which found that ARR values exceeded 70 pmol/mU in 25% of people newly diagnosed with hypertension (mean age, 55 years).19 On the other hand, only two of 758 participants in the Western Australia Raine Study (age, 27 years) had elevated ARR values using the same threshold.20 It is unclear why the prevalence of elevated ARRs was higher among our urban ABC and TEC participants.
The proportions of women among TEC and urban ABC participants with elevated ARR values were larger than of the participants with lower values. Median ARR values are generally higher in women than in men, probably the result of higher oestrogen and progesterone levels and hormonal therapies.21,22 Oral contraceptives can cause false positive primary aldosteronism screening results,23 but 65 of 93 women in the ABC and TEC groups who used contraception used etonogestrel, a subdermal, slow release progestogen not associated with significant ARR changes.23 All three remote ABC participants with elevated ARRs used etonogestrel. As only one of eight women in the urban ABC group and two of seventeen TEC participants with elevated ARR values used oral contraception, it is unlikely to explain the higher proportions of positive test results in these groups.
In each study group, mean blood pressure and the proportions of people with hypertension were similar for participants with ARR values above or no more than 70 pmol/mU; about one‐third of TEC and remote ABC participants and one‐quarter of urban ABC participants with ARRs exceeding 70 pmol/mU had at least grade 1 hypertension. The recognised association between primary aldosteronism and cardiovascular disease, particularly in older people,24,25 may be less evident in younger people in whom the prevalence of clinical hypertension is lower. However, the Raine Study found that higher ARR at 17 years of age was associated with higher blood pressure at 27 years of age.20 Moreover, given evidence for increased risk of incident hypertension in people with low renin and high aldosterone levels,7,26 the relationship between ARR and blood pressure may evolve over time.
The implications of elevated ARR values for people without hypertension have not been investigated. Nevertheless, the large proportion of people in the urban ABC group with positive primary aldosteronism screening test results suggests that more screening of urban Indigenous people would be appropriate, particularly of people with hypertension, for whom primary aldosteronism is associated with adverse cardiovascular outcomes.
Barriers to the diagnosis of primary aldosteronism in Indigenous people in the Northern Territory include the lack of specific screening guidelines,27,28,29 the inadequacy of local diagnostic services (particularly adrenal vein sampling for subtyping of primary aldosteronism), and the risk of renin cryoactivation during sample transport from remote areas. Improving screening for primary aldosteronism in Indigenous Territorians will entail ensuring adequate sample collection and storage, and will be more feasible if undertaken locally and in a culturally sensitive manner.
We found a high positive primary aldosteronism screening test result rate in the Northern Territory, which suggests that the prevalence of primary aldosteronism may be high. In remote areas where undertaking the saline suppression test is difficult, the captopril challenge test could be used as a simpler alternative for confirming primary aldosteronism. In areas where screening itself is impractical, empiric spironolactone could be considered as an inexpensive and effective medication for managing poorly controlled hypertension and the associated risks of cardiovascular and renal disease.
Limitations
Our study is the first to evaluate aldosterone and renin concentrations in Indigenous Australians and to compare them with values for non‐Indigenous people of similar age. Limitations included our lack of information about electrolyte levels, salt consumption, menstrual cycle stage, and medications that can affect aldosterone, renin, and blood pressure assessments (apart from antihypertensive agents, used by seven participants). As aldosterone production can be lower in people with hypokalaemia, the lack of electrolyte data may have led to underestimation of the prevalence of high ARR values. Further, confirmatory testing to establish primary aldosteronism diagnoses had not yet been undertaken, and its prevalence could consequently only be inferred. The prevalence of positive screening test results depends on its definition; the threshold we applied (70 pmol/mU) is typical for Australian practice,13 and no minimum plasma aldosterone concentration was required; this relatively high threshold yields a conservative estimate of the number of participants with possible primary aldosteronism.30 Finally, primary aldosteronism screening was not conducted according to the international Endocrine Society recommendations,31 (ie, we screened all participants, not just those with higher blood pressure or resistant hypertension) as abnormal aldosterone regulation has been identified in some people without hypertension,7,26,32 suggesting that its production can begin to rise prior to the onset of hypertension.6
Conclusion
The ARR was elevated for about one‐quarter of the urban Indigenous and non‐Indigenous participants screened in our study. Following up people with positive primary aldosteronism screening results and determining the relationship between the ARR and future blood pressure levels would be beneficial, as the burden of cardiovascular disease among Indigenous Australians is high and information on the prevalence of primary aldosteronism limited. Indigenous Australians should have the same access to primary aldosteronism screening and diagnosis as other Australians, as this condition may compound the effects of other cardiovascular risk factors for Aboriginal and Torres trait Islander people. A prospective study employing the complete primary aldosteronism diagnosis pathway could better determine its prevalence among Indigenous Australians.
Box 1 – Baseline characteristics of the study participants, by study group
|
Characteristic |
Top End Cohort |
Aboriginal Birth Cohort (urban) |
Aboriginal Birth Cohort (remote) |
||||||||||||
|
|
|||||||||||||||
|
Participants |
76 |
50 |
205 |
||||||||||||
|
Age (years), mean (SD)* |
30.3 (1.5) |
32.0 (1.2) |
32.4 (1.3) |
||||||||||||
|
Sex (women) |
48 (63%) |
22 (44%) |
104 (51%) |
||||||||||||
|
Alcohol use (ever)* |
66 (88%) |
42 (84%) |
90 (46%) |
||||||||||||
|
Alcohol use (weekly)* |
31 (41%) |
24 (48%) |
59 (30%) |
||||||||||||
|
Smoker* |
8 (11%) |
24 (48%) |
154 (78%) |
||||||||||||
|
Height (cm), mean (SD) |
170.7 (10.1) |
170.9 (9.6) |
167.1 (8.2) |
||||||||||||
|
Weight (kg), mean (SD) |
77.6 (18.9) |
88.0 (21.7) |
67.3 (17.4) |
||||||||||||
|
Waist circumference (cm), mean (SD)* |
88.5 (13.1) |
102.0 (19.0) |
89.5 (15.3) |
||||||||||||
|
Body mass index (kg/m2), mean (SD) |
26.5 (5.4) |
30.1 (7.0) |
24.1 (5.9) |
||||||||||||
|
< 25 kg/m2 |
34 (45%) |
13 (26%) |
128 (62%) |
||||||||||||
|
25.0–29.9 kg/m2 |
24 (32%) |
18 (36%) |
38 (19%) |
||||||||||||
|
≥ 30.0 kg/m2 |
18 (24%) |
19 (38%) |
39 (19%) |
||||||||||||
|
Systolic blood pressure (mmHg), mean (SD)* |
117 (13) |
121 (16) |
115 (13) |
||||||||||||
|
Diastolic blood pressure (mmHg), mean (SD)* |
74 (8) |
79 (11) |
75 (9) |
||||||||||||
|
Grade 1 hypertension† |
|
|
|
||||||||||||
|
Yes |
21 (28%) |
18 (36%) |
52 (25%) |
||||||||||||
|
No |
54 (72%) |
32 (64%) |
153 (75%) |
||||||||||||
|
Grade 2 hypertension‡ |
|
|
|
||||||||||||
|
Yes |
5 (7%) |
5 (10%) |
10 (5%) |
||||||||||||
|
No |
70 (93%) |
45 (90%) |
195 (95%) |
||||||||||||
|
Plasma aldosterone concentration (pmol/L), median (IQR) |
273 (182–499) |
312 (248–448) |
259 (199–408) |
||||||||||||
|
Direct renin concentration (mU/L), median (IQR) |
7.5 (4.1–12.4) |
12.4 (5.1–19.0) |
29.3 (15.0–52.9) |
||||||||||||
|
Aldosterone‐to‐renin ratio (pmol/mU), median (IQR) |
43.0 (25.5–73.5) |
28.0 (16.2–69.8) |
10.0 (6.0–19.0) |
||||||||||||
|
≤ 70 |
55 (72%) |
37 (74%) |
199 (97%) |
||||||||||||
|
> 70 |
21 (28%) |
13 (26%) |
6 (3%) |
||||||||||||
|
≤ 50 |
45 (59%) |
34 (68%) |
194 (95%) |
||||||||||||
|
> 50 |
31 (41%) |
16 (32%) |
11 (5%) |
||||||||||||
|
Antihypertensive therapy use |
|
|
|
||||||||||||
|
Yes |
1 (1%) |
1 (2%) |
5 (3%) |
||||||||||||
|
No |
75 (99%) |
49 (98%) |
200 (97%) |
||||||||||||
|
Contraception use (women only)* |
|
|
|
||||||||||||
|
Yes |
15 (34%) |
8 (42%) |
70 (74%) |
||||||||||||
|
No |
29 (66%) |
11 (58%) |
25 (26%) |
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. * Missing data: Top End Cohort: alcohol use and smoking (one person), waist circumference (three), blood pressure (one), contraception use (four); Aboriginal Birth Cohort (urban): waist circumference (one person), contraception use (three); Aboriginal Birth Cohort (remote): age (three people), alcohol intake and smoking (seven people), waist circumference (four), contraception use (nine). † Systolic blood pressure > 130 mmHg or diastolic blood pressure > 80 mmHg. ‡ Systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg. |
|||||||||||||||
Box 2 – Aldosterone and renin concentrations, and aldosterone‐to‐renin ratio, by study group*
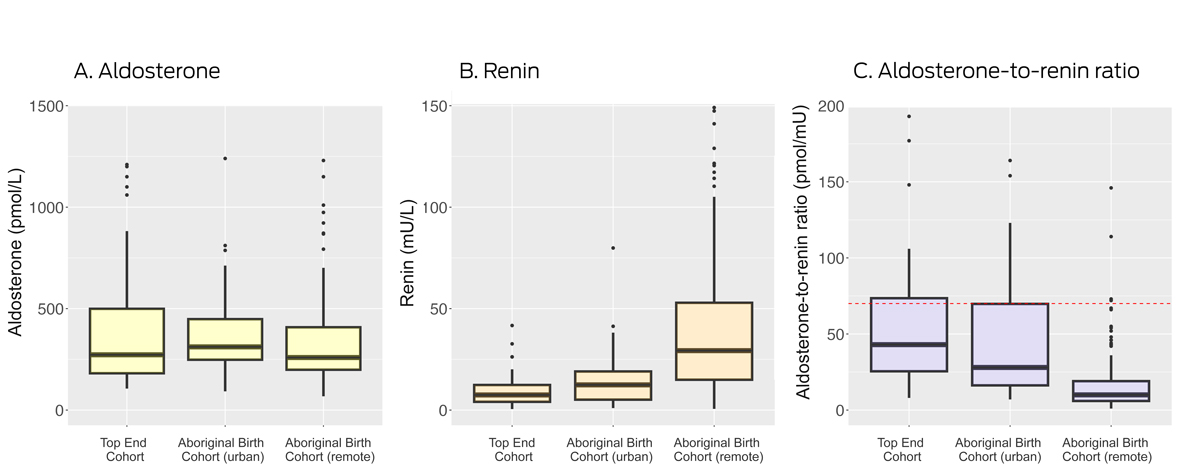
* Boxes depict interquartile range for values (median marked as bar within box; the whiskers depict the range included within 1.5 interquartile ranges; dots depict outliers. Not depicted: aldosterone values above 1500 pmol/L (Top End Cohort: one person; remote Aboriginal Birth Cohort: three people); renin values above 150 mU/L (remote Aboriginal Birth Cohort: five people); ARR values above 200 pmol/mU (Top End Cohort: seven people; urban Aboriginal Birth Cohort: four people; remote Aboriginal Birth Cohort: two people). The red dotted line in panel C marks the threshold (70 pmol/mU) for a positive screening test result.
Box 3 – Aldosterone‐to‐renin ratio, blood pressure, and sex, by study group
|
|
Top End Cohort |
Aboriginal Birth Cohort (urban) |
Aboriginal Birth Cohort (remote) |
||||||||||||
|
Characteristic |
ARR ≤ 70 |
ARR > 70 |
P |
ARR ≤ 70 |
ARR > 70 |
P |
ARR ≤ 70 |
ARR > 70 |
P |
||||||
|
|
|||||||||||||||
|
Participants |
55 |
21 |
|
37 |
13 |
|
199 |
6 |
|
||||||
|
Systolic blood pressure (mmHg), mean (SD) |
116 (12) |
120 (14) |
0.36 |
121 (16) |
121 (16) |
0.84 |
115 (13) |
117 (4) |
0.48 |
||||||
|
Diastolic blood pressure (mmHg), mean (SD) |
73 (8) |
77 (8) |
0.09 |
80 (12) |
79 (11) |
0.64 |
75 (9) |
78 (5) |
0.19 |
||||||
|
Grade 1 hypertension* |
|
|
0.49 |
|
|
0.33 |
|
|
0.65 |
||||||
|
No |
41 (75%) |
14 (67%) |
|
22 (60%) |
10 (77%) |
|
149 (75%) |
4 (67%) |
|
||||||
|
Yes |
14 (25%) |
7 (33%) |
|
15 (41%) |
3 (23%) |
|
50 (25%) |
2 (33%) |
|
||||||
|
Grade 2 hypertension† |
|
|
0.13 |
|
|
> 0.99 |
|
|
> 0.99 |
||||||
|
No |
53 (96%) |
18 (86%) |
|
33 (89%) |
12 (92%) |
|
189 (95%) |
6 (100%) |
|
||||||
|
Yes |
2 (4%) |
3 (14%) |
|
4 (11%) |
1 (8%) |
|
10 (5%) |
0 |
|
||||||
|
Sex |
|
|
0.047 |
|
|
0.14 |
|
|
> 0.99 |
||||||
|
Men |
24 (44%) |
4 (19%) |
|
23 (62%) |
5 (39%) |
|
98 (49%) |
3 (50%) |
|
||||||
|
Women |
31 (56%) |
17 (81%) |
|
14 (38%) |
8 (62%) |
|
101 (51%) |
3 (50%) |
|
||||||
|
Contraception use (women only)* |
|
|
0.21 |
|
|
0.35 |
|
|
0.56 |
||||||
|
Yes |
8 (28%)‡ |
7 (47%)‡ |
|
6 (55%)¶ |
2 (25%)** |
|
67 (73%)†† |
3 (100%)‡‡ |
|
||||||
|
No |
21 (72%) |
8 (53%) |
|
5 (46%) |
6 (75%) |
|
25 (27%) |
0 |
|
||||||
|
Missing data |
2 |
2 |
|
3 |
0 |
|
9 |
0 |
|
||||||
|
|
|||||||||||||||
|
ARR = aldosterone‐to‐renin ratio (pmol/mU); SD = standard deviation. * Systolic blood pressure > 130 mmHg or diastolic blood pressure > 80 mmHg. † Systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg. ‡ Etonogestrel (Implanon), one; medroxyprogesterone (Depo‐Provera), two; oral contraception, two; other, three. § Etonogestrel, one; oral contraception, two; not specified, four. ¶ Etonogestrel, three; medroxyprogesterone, one; oral contraception, two. ** Medroxyprogesterone, one; oral contraception, one. †† Etonogestrel, 57; oral contraception, four; other, two; unspecified, four. ‡‡ Etonogestrel, three. |
|||||||||||||||
Box 4 – Blood pressure in study participants, by study group and participant characteristics: multivariable linear regression analyses
|
|
Systolic blood pressure (mmHg) |
Diastolic blood pressure (mmHg) |
|||||||||||||
|
Characteristic |
Mean (SD) |
Beta (95% CI)* |
Mean (SD) |
Beta (95% CI)* |
|||||||||||
|
|
|||||||||||||||
|
Top End Cohort |
|
|
|
|
|||||||||||
|
Age (per year) |
— |
–1.0 (–2.6 to 0.7) |
— |
–0.04 (–1.2 to 1.1) |
|||||||||||
|
Sex (men) |
124.8 (13.2) |
1 |
76.7 (8.5) |
1 |
|||||||||||
|
Sex (women) |
112.1 (9.9) |
–13.2 (–8.0 to –8.3) |
72.9 (7.8) |
–4.1 (–7.9 to –0.3) |
|||||||||||
|
Waist circumference (per cm) |
— |
0.4 (0.2 to 0.6) |
— |
0.4 (0.2 to 0.5) |
|||||||||||
|
Body mass index, (per 1.0 kg/m2) |
— |
0.9 (0.5 to 1.3) |
— |
0.8 (0.5 to 1.1) |
|||||||||||
|
< 25 kg/m2 |
112.7 (13.5) |
1 |
70.6 (7.3) |
1 |
|||||||||||
|
25.0–29.9 kg/m2 |
118.2 (11.4) |
3.0 (–2.7 to 8.8) |
75.6 (8.1) |
4.3 (0.3 to 8.3) |
|||||||||||
|
≥ 30.0 kg/m2 |
122.4 (10.9) |
10.9 (4.6 to 17.1) |
79.4 (7.0) |
9.1 (4.7 to 13.4) |
|||||||||||
|
Plasma aldosterone (per 1.0 pmol/L) |
— |
0.000 (–0.007 to 0.007) |
— |
0.002 (–0.003 to 0.007) |
|||||||||||
|
Direct renin (per 1.0 mU/L) |
— |
–0.3 (–0.6 to 0.0) |
— |
–0.1 (–0.3 to 0.2) |
|||||||||||
|
Aldosterone‐to‐renin ratio (per 1.0 pmol/mU) |
— |
0.04 (0.01 to 0.07) |
— |
0.02 (0.00 to 0.04) |
|||||||||||
|
Aboriginal Birth Cohort (urban) |
|
|
|
|
|||||||||||
|
Age (per year) |
— |
0.2 (–3.4 to 3.7) |
— |
0.7 (–1.9 to 3.2) |
|||||||||||
|
Sex (men) |
126.0 (17.8) |
1 |
82.3 (13.2) |
1 |
|||||||||||
|
Sex (women) |
113.9 (9.7) |
–13 (–21.2 to –4.7) |
75.9 (7.1) |
–6.9 (–12.9 to –0.9) |
|||||||||||
|
Waist circumference (per cm) |
— |
0.3 (0.1 to 0.5) |
— |
0.3 (0.1 to 0.4) |
|||||||||||
|
Body mass index (per 1.0 kg/m2) |
— |
0.7 (0.1 to 1.3) |
— |
0.6 (0.2 to 1.0) |
|||||||||||
|
< 25 kg/m2 |
113.2 (12.0) |
1 |
73.4 (9.0) |
1 |
|||||||||||
|
25.0–29.9 kg/m2 |
121.0 (11.3) |
6.2 (–4.3 to 16.7) |
78.6 (7.5) |
4.2 (–3.3 to 11.7) |
|||||||||||
|
≥ 30.0 kg/m2 |
125.5 (20.0) |
13.2 (2.9 to 23.5) |
84.4 (13.7) |
11.5 (4.1 to 18.9) |
|||||||||||
|
Plasma aldosterone (per 1.0 pmol/L) |
— |
–0.008 (–0.028 to 0.013) |
— |
–0.004 (–0.019 to 0.011) |
|||||||||||
|
Direct renin (per 1.0 mU/L) |
— |
–0.2 (–0.5 to 0.1) |
— |
–0.1 (–0.3 to 0.1) |
|||||||||||
|
Aldosterone‐to‐renin ratio (per 1.0 pmol/mU) |
— |
0.03 (–0.02 to 0.08) |
— |
0.02 (–0.02 to 0.06) |
|||||||||||
|
Aboriginal Birth Cohort (remote) |
|
|
|
|
|||||||||||
|
Age (per year) |
— |
0.9 (–0.4 to 2.3) |
— |
1.1 (0.1 to 2.0) |
|||||||||||
|
Sex (men) |
119.2 (12.9) |
1 |
76.8 (8.9) |
1 |
|||||||||||
|
Sex (women) |
111.3 (12.2) |
–8.5 (–11.8 to –5.1) |
73.5 (8.2) |
–3.7 (–6.0 to –1.4) |
|||||||||||
|
Waist circumference (per cm) |
— |
0.2 (0.1 to 0.3) |
— |
0.1 (0.1 to 0.2) |
|||||||||||
|
Body mass index (per 1.0 kg/m2) |
— |
0.6 (0.3 to 0.9) |
— |
0.4 (0.2 to 0.6) |
|||||||||||
|
< 25 kg/m2 |
113.0 (11.9) |
1 |
73.3 (8.1) |
1 |
|||||||||||
|
25.0–29.9 kg/m2 |
119.8 (16.1) |
8.2 (3.7 to 12.7) |
78.6 (8.6) |
5.8 (2.8 to 8.8) |
|||||||||||
|
≥ 30.0 kg/m2 |
118.1 (12.2) |
5.8 (1.3 to 10.2) |
77.7 (9.3) |
4.5 (1.5 to 7.5) |
|||||||||||
|
Plasma aldosterone (per 1.0 pmol/L) |
— |
0.002 (–0.004 to 0.009) |
— |
0.002 (–0.002 to 0.006) |
|||||||||||
|
Direct renin (per 1.0 mU/L) |
— |
0.011 (–0.020 to 0.042) |
— |
0.001 (–0.020 to 0.022) |
|||||||||||
|
Aldosterone‐to‐renin ratio (per 1.0 pmol/mU) |
— |
0.01 (–0.02 to 0.04) |
— |
0.01 (–0.02 to 0.03) |
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; SD = standard deviation. Bold: Statistically significant. * Age, sex: adjusted for age or sex (as applicable) and body mass index; body mass index and weight circumference adjusted for age and sex; aldosterone, renin, aldosterone‐to‐renin ratio: adjusted for age, sex, and body mass index. The beta values for unadjusted analyses are reported in the Supporting Information. |
|||||||||||||||
Received 1 February 2023, accepted 20 June 2023
- Elisabeth Ng1,2
- Stella M Gwini2,3
- Michael Stowasser4
- Morag J Young5
- Peter J Fuller1,2
- Gurmeet R Singh6
- Jun Yang1,2
- 1 Monash Health, Melbourne, VIC
- 2 Centre for Endocrinology and Metabolism, Hudson Institute of Medical Research, VIC
- 3 Monash University, Melbourne, VIC
- 4 Princess Alexandra Hospital, Brisbane, QLD
- 5 Baker Heart and Diabetes Institute, Melbourne, VIC
- 6 Menzies School of Health Research, Darwin, NT
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
The aldosterone and renin test kits were provided by DiaSorin. The Aboriginal Birth Cohort study is funded by an National Health and Medical Research Council project grant (APP1138609). Jun Yang is supported by an NHMRC Investigator Grant. Morag J Young is supported by an Alice Baker and Eleanor Shaw Gender Equity Fellowship. Elisabeth Ng is supported by an NHMRC and Heart Foundation PhD Scholarship and a Royal Australasian College of Physicians Vincent Fairfax Family Foundation Research Entry Scholarship. The Hudson Institute of Medical Research and Baker Heart and Diabetes Institute are supported by the Operational Infrastructure Scheme of the Victorian government. Funders did not have any role in study design, data collection, analysis or interpretation, reporting or publication. We acknowledge Monash Pathology for determining plasma aldosterone and direct renin concentrations.
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Cardiovascular disease, diabetes and chronic kidney disease. Australian facts: Aboriginal and Torres Strait Islander people 2015. 25 Nov 2015. https://www.aihw.gov.au/reports/heart‐stroke‐vascular‐disease/cardiovascular‐diabetes‐chronic‐kidney‐indigenous/summary (viewed Dec 2022).
- 2. Australian Institute of Health and Welfare. High blood pressure. In: Aboriginal and Torres Strait Islander health performance framework; 2020 summary report (Cat. no. IHPF 2); pp. 39‐40. Canberra: AIHW, 2020. https://www.indigenoushpf.gov.au/getattachment/65fbaaf3‐100c‐4df5‐941c‐a8455922693c/2020‐summary‐ihpf‐2.pdf (viewed Jan 2023).
- 3. Li M, McDermott R. Obesity, albuminuria, and gamma‐glutamyl transferase predict incidence of hypertension in indigenous Australians in rural and remote communities in northern Australia. J Hypertens 2015; 33: 704‐710.
- 4. Brown A, O'Shea RL, Mott K, et al; Essential Service Standards for Equitable National Cardiovascular Care for Aboriginal and Torres Strait Islander people (ESSENCE) Steering Committee. Essential service standards for equitable national cardiovascular care for Aboriginal and Torres Strait Islander people. Heart Lung Circ 2015; 24: 126‐141.
- 5. Funder JW, Carey RM. Primary aldosteronism: Where are we now? Where to from here? Hypertension 2022; 79: 726‐735.
- 6. Turcu AF, Yang J, Vaidya A. Primary aldosteronism: a multidimensional syndrome. Nat Rev Endocrinol 2022; 18: 665‐682.
- 7. Brown JM, Robinson‐Cohen C, Luque‐Fernandez MA, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med 2017; 167: 630‐641.
- 8. Hundemer GL, Baudrand R, Brown JM, et al. Renin phenotypes characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab 2017; 102: 1835‐1843.
- 9. Kathiresan S, Larson MG, Benjamin EJ, et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens. 2005; 18: 657‐665.
- 10. Sayers SM, Mackerras D, Singh G, et al. An Australian Aboriginal birth cohort: a unique resource for a life course study of an Indigenous population. A study protocol. BMC Int Health Hum Rights 2003; 3: 1.
- 11. Sayers S, Singh G, Mackerras D, et al. Australian Aboriginal Birth Cohort study: follow‐up processes at 20 years. BMC Int Health Hum Rights 2009; 9: 23.
- 12. Davison B, Cunningham T, Singh G. Engaging adolescents and young adults in a longitudinal health study: experience from the Top End cohort. Aust N Z J Public Health 2011; 35: 86‐87.
- 13. Naruse M, Murakami M, Katabami T, et al. International multicenter survey on screening and confirmatory testing in primary aldosteronism. Eur J Endocrinol 2023; 188: lvac002.
- 14. Sawyer N, Glendenning P, Vasikaran SD, et al. The Adrenal Vein Sampling Outcomes Study (AVOS): success rates following adrenalectomy for unilateral primary aldosteronism. Pathology 2023; 55: 531‐537.
- 15. Liu Z, Deng X, Luo L, et al. Diagnostic value of aldosterone to renin ratio calculated by plasma renin activity or plasma renin concentration in primary aldosteronism: a meta‐analysis. Chin Med J (Engl) 2022; 135: 639‐647.
- 16. Campbell DJ, Nussberger J, Stowasser M, et al. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem 2009; 55: 867‐877.
- 17. Bonnitcha P, Rigdwell M, Ward P, et al. Standard –20°C freezer storage protocols may cause substantial plasma renin cryoactivation. Clin Chem Lab Med 2023; 61:1428‐1435.
- 18. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev 2016; 96: 1327‐1384.
- 19. Libianto R, Russell GM, Stowasser M, et al. Detecting primary aldosteronism in Australian primary care: a prospective study. Med J Aust 2022; 216: 408‐412. https://www.mja.com.au/journal/2022/216/8/detecting‐primary‐aldosteronism‐australian‐primary‐care‐prospective‐study
- 20. Yang J, May Gwini S, Beilin LJ, et al. Relationship between the aldosterone‐to‐renin ratio and blood pressure in young adults: a longitudinal study. Hypertension 2021; 78: 387‐396.
- 21. Ahmed AH, Gordon RD, Taylor PJ, et al. Are women more at risk of false‐positive primary aldosteronism screening and unnecessary suppression testing than men? J Clin Endocrinol Metab 2011; 96: E340‐E346.
- 22. Ahmed AH, Gordon RD, Ward G, et al. Effect of combined hormonal replacement therapy on the aldosterone/renin ratio in postmenopausal women. J Clin Endocrinol Metab 2017; 102: 2329‐2334.
- 23. Ahmed AH, Gordon RD, Taylor PJ, et al. Effect of contraceptives on aldosterone/renin ratio may vary according to the components of contraceptive, renin assay method, and possibly route of administration. J Clin Endocrinol Metab 2011; 96: 1797‐1804.
- 24. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2018; 6: 41‐50.
- 25. Mulatero P, Monticone S, Bertello C, et al. Long‐term cardio‐ and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 2013; 98: 4826‐4833.
- 26. Arnold N, Hermanns IM, Schulz A, et al. Renin, aldosterone, the aldosterone‐to‐renin ratio, and incident hypertension among normotensive subjects from the general population. Cardiovasc Res 2023; 119: 294‐301.
- 27. National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. 2012. https://informme.org.au/media/cuzjrcgz/absolutecvd_gl_webready.pdf (viewed Jan 2023).
- 28. Central Australian Rural Practitioners Association. CARPA standard treatment manual. 8th edition. Alice Springs: Flinders University, 2022. https://www.remotephcmanuals.com.au/download/39290?filename=RPHCM_STM_Manual_8thEd.pdf (viewed Mar 2023).
- 29. National Aboriginal Community Controlled Health Organisation; Royal Australian College of General Practitioners. National guide to a preventive health assessment for Aboriginal and Torres Strait Islander people. 3rd edition. Melbourne: RACGP, 2018. https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Resources/National‐guide‐3rd‐ed‐Sept‐2018‐web.pdf (viewed Jan 2023).
- 30. Stowasser M, Gordon RD. Primary aldosteronism. Best Pract Res Clin Endocrinol Metab 2003; 17: 591‐605.
- 31. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 1889‐1916.
- 32. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross‐sectional study. Ann Intern Med 2020; 173: 10‐20.






Abstract
Objectives: To evaluate aldosterone and renin levels and aldosterone‐to‐renin ratios (ARRs) in young Indigenous and non‐Indigenous adults in the Northern Territory, and their association with blood pressure levels.
Design: Cross‐sectional study; single time point sub‐study of two prospective birth cohort studies.
Setting, participants: Participants in the Aboriginal Birth Cohort (ABC) — born to Indigenous mothers at the Royal Darwin Hospital during 1987–1990 — and the Top End Cohort (TEC) — people born to non‐Indigenous mothers in Darwin, recruited during 2007–2009 — aged 32–35 years at the time of this sub‐study.
Main outcome measures: Plasma aldosterone and direct renin concentrations; ARRs (positive screening test result for primary aldosteronism defined as > 70 pmol/mU); systolic and diastolic blood pressure.
Results: A total of 255 ABC (205 in remote, 50 in urban locations) and 76 TEC members participated. Median aldosterone concentration was similar for all three groups. The median renin concentration was 7.5 mU/L (interquartile range [IQR], 4.1–12.4 mU/L) in the TEC group, 12.4 mU/L (IQR, 5.1–19 mU/L) in the urban ABC group, and 29.3 mU/L (IQR, 15.0–52.9 mU/L) in the remote ABC group. The median ARR was 10 pmol/mU (IQR, 6–19 pmol/mU) in the remote ABC group, 28 pmol/mU (IQR, 16–70 pmol/mU) in the urban ABC group, and 43 pmol/mU (IQR, 26–74 pmol/mU) in the TEC group. Thirteen urban ABC participants (26%), 21 TEC participants (28%), and six people in the remote ABC group (3%) had ARR values above 70 pmol/mU. Adjusted for age and body mass index (BMI), mean systolic and diastolic blood pressure were lower for women than men in all participant groups; after adjusting for age, sex, and BMI, larger ARR was associated with higher systolic blood pressure in the TEC group but not the two ABC groups.
Conclusion: Screening test results for primary aldosteronism were positive for about one‐quarter of urban Indigenous and non‐Indigenous participants. A prospective study that includes confirmatory testing would more accurately assess the prevalence of primary aldosteronism among Indigenous Australians in the Northern Territory.