Clinical record
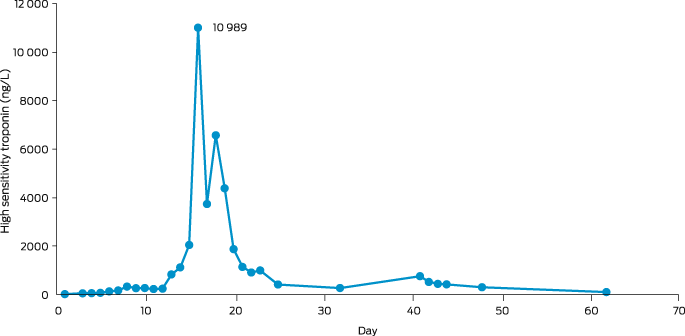
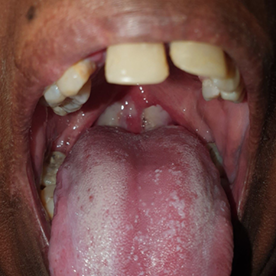
A 32‐year‐old woman from a remote Indigenous community in Far North Queensland presented to her local health clinic with a one‐day history of odynophagia. She had suboptimally controlled type 2 diabetes mellitus, reported no recent travel, and had received five diphtheria‐containing vaccines during childhood and adolescence, the last being 13 years ago. Streptococcal pharyngitis was suspected and she was prescribed oral phenoxymethylpenicillin, but despite good adherence, she represented two days later with worsening symptoms. She received intravenous amoxicillin–clavulanate and, due to concerns about developing stridor, was administered dexamethasone to decrease upper airway swelling. She was transferred to our hospital and, on arrival, was dysphonic and had a markedly swollen neck with whitish–grey membranous plaques covering her tonsils, faucial pillars and soft palate (Box 1). Cardiac and neurological examinations were normal, and electrocardiogram (ECG) showed sinus rhythm. Respiratory diphtheria was suspected. A throat swab was collected, the microbiology laboratory notified, diphtheria antitoxin was requested from the nearest pharmacy where it was available (1600 km away), intravenous amoxicillin–clavulanate and azithromycin were commenced, and the patient was managed with standard plus droplet precautions. The local public health team were notified, and contact identification commenced immediately. The following day, the patient received 100 000 units of diphtheria antitoxin intravenously without complication. Corynebacterium diphtheriae was identified from the patient's throat swab 72 hours after collection; toxigenic C. diphtheriae was confirmed by polymerase chain reaction (PCR). Susceptibility testing revealed intermediate susceptibility to penicillin and susceptibility to clindamycin. Blood collected on admission revealed an antidiphtheria toxoid IgG level of 0.55 IU/mL (protective level, > 0.1 IU/mL). By day 3, the patient's odynophagia and neck swelling were improving, but an ECG identified a prolonged corrected QT interval (497 milliseconds) and serum cardiac troponin I was 44 ng/L (reference interval, < 10 ng/L). Her antibiotics were changed to lincomycin, her odynophagia improved, and repeat throat swabs were culture negative after 14 days of therapy. However, despite minimal chest pain or breathlessness, her serum troponin continued to rise and on day 16 was 10 989 ng/L (Box 2). A transthoracic echocardiogram was normal but cardiac magnetic resonance imaging identified subepicardial inflammation in the inferior wall consistent with myocarditis. A coronary angiogram was normal. She was discharged from hospital on day 25 and received a diphtheria–tetanus vaccine 33 days after diphtheria antitoxin was administered.
On day 40, the patient represented with dizziness, cough, perioral and buttock paraesthesia, dysphagia and recurrent odynophagia. A throat swab identified influenza A and rhinovirus; C. diphtheriae was not isolated. Flexible nasoendoscopy found an erythematous, oedematous nasopharynx with normal epiglottis and symmetrical, mobile vocal cords. Her symptoms persisted, and, on day 48, she developed hypoxia, worsening dysphagia, dysarthria and symmetrical facial weakness. Repeat flexible nasoendoscopy identified absent supraglottic sensation, near‐complete left and partial right vocal cord paralysis. Chest imaging confirmed pneumonia. She was kept nil by mouth, and video fluoroscopy confirmed sensorimotor oropharyngeal dysphagia with silent aspiration; a nasogastric tube was placed for nutritional support. Impaired airway clearance necessitated twice‐daily chest physiotherapy with high flow oxygen, cough‐assist device, and self‐directed nasopharyngeal suctioning. On day 54, she underwent a percutaneous endoscopic transgastric jejunostomy and had a tracheostomy on day 57. The patient had regular fibreoptic endoscopic evaluations of swallowing and, by day 69, her vocal cord sensation and movement had improved. Over the next 12 days, she had progressive lengthening of her tracheostomy cuff deflation and transitioned from thickened fluids and pureed diet to normal meals. Her larynx recovered fully, and the tracheostomy was removed on day 81. She was discharged on day 84 (Box 3).
Contact tracing identified five close contacts. They were advised to monitor for symptoms, underwent nasal, throat and skin lesion swabs; received oral azithromycin; received vaccination (if required); and were advised not to interact with others until completion of 72 hours or more of antibiotics while swab results were pending. None returned a positive swab or developed symptoms. A public health alert was disseminated to local health care workers and opportunistic diphtheria vaccination was encouraged for eligible individuals.
Discussion
Respiratory diphtheria classically begins as an upper respiratory tract infection. Local toxin production causes a pseudomembrane to form on the pharynx or larynx and can cause a markedly swollen “bull” neck. If diphtheria is suspected, diphtheria antitoxin should be given as soon as possible as culture results can be delayed.1 Absorption of diphtheria toxin can lead to cardiac, neurological and renal complications. Cardiac toxicity may occur acutely but often occurs seven to 14 days after respiratory symptom onset; neurological involvement, particularly bulbar weakness and other cranial neuropathies, may not appear for weeks to months.2 Neurological manifestations of diphtheria usually resolve completely3 but require supportive, multidisciplinary care.
Widespread vaccination means that toxigenic diphtheria is now rare in Australia, but cases still occur, usually in returned travellers or in unvaccinated or partially vaccinated people.4 However, locally acquired cases have recently been reported.5,6,7 Vaccination with five doses of a diphtheria‐containing vaccine is up to 99% effective in preventing symptomatic disease and a three‐dose primary series plus two to three booster doses should provide adequate protection throughout adolescence and adulthood.1,8 As immunity can wane over time, some countries recommend a booster for adults every ten years.9 In Australia, booster doses are recommended for adolescents, adults aged 50 years and older, laboratory workers and travellers to endemic areas.10
It is unclear why our patient had a complicated course. Her antibody levels were above those required for protection,11 although these were collected after she had developed symptoms and may have been lower before disease onset. Our patient's suboptimally controlled type 2 diabetes mellitus may have predisposed her to developing more invasive disease.12
High childhood and adolescent vaccination rates and the rarity of cases suggests that outbreaks of toxigenic diphtheria are unlikely in Far North Queensland, but this may change if vaccine uptake declines. Sporadic cases may continue to occur in well vaccinated populations, and it remains important to investigate locally acquired cases. However, more detailed investigations including seroprevalence studies and determination of toxigenic C. diphtheriae carriage rates may be required before considering any change to current Australian vaccine recommendations.
Lessons from practice
- Respiratory diphtheria is now rare in Australia, but locally acquired cases are increasing; the recommended frequency of adult booster doses may need to be re‐evaluated if high risk community settings emerge.
- Vaccine‐induced immunity can wane over time and booster doses are essential to maintain protective immunity.
- Diagnosing respiratory diphtheria clinically requires a high index of suspicion; diphtheria antitoxin should be given as soon possible if the diagnosis is suspected.
- Respiratory diphtheria often follows a complicated course, with delayed onset cardiac and neurological involvement that requires patient counselling, close monitoring, and multidisciplinary supportive care.
Box 2 – Troponin trend over time in patient with cardiac toxicity secondary to respiratory diphtheria from first admission to our hospital
Box 3 – Time course of management in patient with cardiac and neurological manifestations of respiratory diphtheria
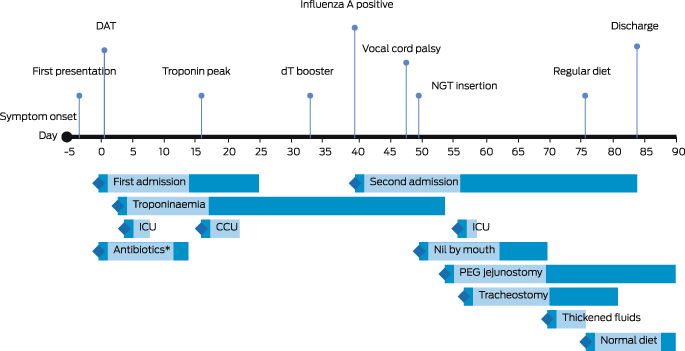
DAT = diphtheria antitoxin; dT = diphtheria–tetanus combination vaccine; NGT = nasogastric tube; ICU = intensive care unit admission; CCU = coronary care unit admission; PEG = percutaneous endoscopic gastrostomy. * Antibiotics active against Corynebacterium diphtheriae.
Provenance: Not commissioned; externally peer reviewed.
- 1. UK Health Security Agency. Public health control and management of diphtheria in England 2022 guidelines. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1117027/diphtheria‐guidelines‐2022_v17_111122.pdf (viewed Feb 2023).
- 2. Truelove SA, Keegan LT, Moss WJ, et al. Clinical and epidemiological aspects of diphtheria: a systematic review and pooled analysis. Clin Infec Dis 2020; 71: 89‐97.
- 3. Prasad PL, Rai PL. Prospective study of diphtheria for neurological complications. J Pediatr Neurosci 2018; 13: 313‐316.
- 4. Grigg S, Hogan D, Hosein FS, et al. A case of toxigenic, pharyngeal diphtheria in Australia. Med J Aust 2020; 213: 64‐55. https://www.mja.com.au/journal/2020/213/2/case‐toxigenic‐pharyngeal‐diphtheria‐australia
- 5. Winkler NE, Dey A, Quinn HE, et al. Australian vaccine preventable disease epidemiological review series: diphtheria 1999–2019. Commun Dis Intell (2018) 2022; 46.
- 6. Beard F, Macartney K, Winkler N. Diphtheria is back in Australia, here's why – and how vaccines can prevent its spread. The Conversation 2022; 5 July. https://theconversation.com/diphtheria‐is‐back‐in‐australia‐heres‐why‐and‐how‐vaccines‐can‐prevent‐its‐spread‐186348 (viewed Nov 2022).
- 7. Hempenstall A, Short J, Marquardt T, et al. Clinician alert: toxigenic diphtheria cases across North Queensland are on the rise. Med J Aust 2023; 218: 238. https://www.mja.com.au/journal/2023/218/5/clinician‐alert‐toxigenic‐diphtheria‐cases‐across‐north‐queensland‐are‐rise
- 8. Diphtheria vaccine: WHO position paper — August 2017. Wkly Epidemiol Rec 2017; 92: 417‐435.
- 9. Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69: 77‐83.
- 10. Australian Technical Advisory Group on Immunisation (ATAGI). Australian immunisation handbook. Canberra: Commonwealth of Australia, 2022. https://immunisationhandbook.health.gov.au/ (viewed Nov 2022).
- 11. Sutter RW, Hardy IR, Kozlova IA, et al. Immunogenicity of tetanus‐diphtheria toxoids (Td) among Ukrainian adults: implications for diphtheria control in the Newly Independent States of the Former Soviet Union. J Infect Dis 2000; 181 (Suppl): S197‐S202.
- 12. Romney MG, Roscoe DL, Bernard K, et al. Emergence of an invasive clone of nontoxigenic Corynebacterium diphtheriae in the urban poor population of Vancouver, Canada. J Clin Microbiol 2006; 44: 1625‐1629.






Open access:
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Patient consent:
The patient gave written consent for publication.
No relevant disclosures.