The known: People with primary aldosteronism have poorer cardiovascular disease outcomes than people with essential hypertension matched for blood pressure. Prompt diagnosis is crucial because targeted treatment can mitigate some of its effects, but people with hypertension are often not screened for primary aldosteronism because it is considered a rare condition.
The new: Screening people with newly diagnosed hypertension by general practitioners before commencing antihypertensive treatment led to the diagnosis of primary aldosteronism in 14% of screened patients.
The implications: GPs could play an important role by actively screening for primary aldosteronism, facilitating early treatment of this readily managed form of secondary hypertension.
Primary aldosteronism (PA) is an important cause of secondary hypertension. In people with PA, autonomous aldosterone secretion by the adrenal gland is inappropriate with respect to sodium status, suppressing renin secretion and resulting in an elevated plasma aldosterone‐to‐renin ratio.1 PA may cause hypokalaemia, but blood potassium levels are normal in about 70% of people with PA.2 Cardiovascular outcomes are poorer for patients with PA than for blood pressure‐matched patients with essential hypertension.3 Adrenalectomy (for people with unilateral adrenal disease) or mineralocorticoid receptor antagonist therapy (for those with bilateral disease, and people with unilateral disease who cannot undergo surgery), can optimise blood pressure control and mitigate many of the adverse cardiovascular consequences of excessive aldosterone secretion, especially if commenced early in the course of disease.4,5
However, only a minority of patients with PA receive targeted treatment, chiefly because the condition is often not recognised and diagnosed.6 For example, a study in northern Italy found that the number of patients discharged from hospital with diagnosis codes consistent with PA corresponded to only 2% of the expected number.7 Our Endocrine Hypertension Service (Monash Health, Melbourne) has reported that the median time between diagnosis with hypertension and referral to the specialist service was 13.5 years, and that 26 of 62 patients with confirmed PA (42%) had end‐organ damage at the time of referral.8 A survey of general practitioners in Victoria found that fewer than 0.1% of about 7000 patients with hypertension had been diagnosed with PA.9 Investigations in primary care suggest that its prevalence among patients with hypertension lies in the range 1–13%.10,11,12,13,14,15,16,17,18,19,20,21
GP‐led screening for PA has not been investigated in Australian primary care. To inform the future investigation and management of hypertension in primary care, we assessed the identification of PA in newly diagnosed, treatment‐naïve patients with hypertension through active screening by GPs. We hypothesised that GP‐led screening prior to the initiation of antihypertensive treatment (which could affect screening results) would identify patients with PA who had previously not been diagnosed with the condition. Our secondary objectives were to assess the characteristics of patients with PA and to evaluate the outcomes of treatment.
Methods
All general practices in the South Eastern Melbourne Primary Health Network with at least three GPs, and all general practices elsewhere in Victoria, that referred patients to the Endocrine Hypertension Clinic at Monash Health during 1 January 2017 – 31 December 2020 were invited during 2017 (practices outside the South Eastern Melbourne Primary Health Network: 2017–2019) to participate in our prospective study. Participating GPs were asked to screen for PA by assessing the aldosterone‐to‐renin ratio (ARR) for people aged 18–80 years with newly diagnosed hypertension (measurements of systolic blood pressure exceeding 140 mmHg or diastolic blood pressure exceeding 90 mmHg on two or more separate occasions) who were not taking antihypertensive medications. Each participating GP received a 30‐minute education session delivered by author JY, and screening was assisted by providing pre‐filled pathology request forms.
Prospective participants received an information sheet about the study, and those who agreed to participate provided written consent for the results of their screening tests and relevant clinical information being forwarded to the research team.
Initial screening for primary aldosteronism
The ARR was assessed at the same time as other routine blood tests (eg, electrolyte levels, renal function assessment). A positive screening test was defined by an ARR of 70 pmol/mU or more (sensitivity and specificity each greater than 95%).22 Patients with an initial positive screening result were referred to the Endocrine Hypertension Clinic, a specialist clinic that investigates and manages patients with endocrine causes of hypertension.8 Patients with negative screening results were deemed to not have PA and their GP continued to manage their hypertension as usual.
For the laboratory assessment of plasma aldosterone and renin concentrations, patients were instructed to have blood samples collected between 8 am and 10 am and after they had been upright for at least two hours. Plasma aldosterone and direct renin concentrations were measured in chemiluminescent immunoassays (LIAISON, DiaSorin). For the aldosterone assay, the within‐run coefficients of variation were 3.5% at 188 pmol/L and 1.8% at 798 pmol/L; total coefficients of variation were 9.6% at 188 pmol/L and 5.6% at 798 pmol/L. For the direct renin assay, the within‐run coefficients of variation were 6.6% at 24.0 mU/L and 1.4% at 92.4 mU/L; total coefficients of variation were 10.0% at 24.0 mU/L and 4.5% at 92.4 mU/L.
Diagnosis and treatment of primary aldosteronism
The ARR was assessed a second time for all patients with positive screening test results who agreed to be referred to the Endocrine Hypertension Clinic for further investigations. Those with a second abnormal ARR value proceeded to confirmatory diagnostic testing, the saline suppression test, in which two litres of physiological saline are infused over four hours (9 am – 1 pm). Prior to June 2019, the diagnosis of PA was deemed confirmed if the plasma aldosterone concentration remained above 140 pmol/L after recumbent saline suppression testing.1 In June 2019, our centre switched to saline suppression testing in the seated position for its greater sensitivity,23 with a plasma aldosterone concentration of 170 pmol/L the threshold for confirming PA. Participating patients who required antihypertensive medication at this point were prescribed medications that would not interfere with PA testing, such as non‐dihydropyridine calcium channel blockers, alpha blockers, hydralazine, or moxonidine.1
Adrenal computed tomography (CT) imaging and adrenal vein sampling (AVS) were offered to patients diagnosed with PA who were suitable candidates for laparoscopic adrenalectomy, to subtype their PA as unilateral or bilateral. Samples were collected from the adrenal veins both before and after administering adrenocorticotropic hormone (ACTH; 250 µg intravenous bolus followed by 50 µg/h infusion24). AVS results were interpreted in accordance with the Endocrine Society guideline.1
Patients with unilateral PA were offered unilateral laparoscopic adrenalectomy. They were assessed after the procedure according to the Primary Aldosteronism Surgical Outcome (PASO) consensus criteria.5 Patients with bilateral PA, unsuccessful AVS, or indeterminate lateralisation, and those who did not undergo AVS were treated with a mineralocorticoid receptor antagonist titrated to optimise blood pressure (below 130/80 mmHg) and to normalise plasma renin (greater than 15 mU/L).25
Statistical analyses
We report the prevalence of PA (number of patients with confirmed PA divided by the number screened) with a 95% confidence interval (CI; Clopper–Pearson binomial proportion interval). Assuming an underlying population prevalence of 5%,6 203 patients were needed to determine the prevalence of PA with 3% precision and 95% confidence. We assessed the normality of continuous data with the Kolmogorov–Smirnov test. The statistical significance of between‐group differences was assessed in Student t tests or one‐way analysis of variance with post hoc Bonferroni correction (normally distributed data), or in Mann–Whitney or Kruskal–Wallis tests (non‐normally distributed data). Categorical data were summarised as frequencies and proportions, and differences assessed in χ2 tests. P < 0.05 (two‐sided) was deemed statistically significant. Statistical analyses were undertaken in SPSS Statistics for Windows 26 (IBM).
Ethics approval
The study was approved by the Monash Health Human Research Ethics Committee (HREC/16/MonH/390).
Results
We contacted 80 GP practices; 31 agreed to participate: 29 in Greater Melbourne and two in regional Victoria. The median practice size was ten GPs (interquartile range [IQR], 7–13 GPs). Seventy GPs recruited patients for the study; six recruited more than ten patients each, 34 recruited one patient each. A total of 256 patients were screened during March 2017 – November 2020, of whom nine were excluded because they were not treatment‐naïve; 62 of 247 eligible patients (25%) had initial positive screening results (ARR ≥ 70 pmol/mU) (Box 1, Box 2).
Eight patients with positive screening results declined further investigations (ie, were not followed up at the Endocrine Hypertension Clinic), and one with two positive ARR results could not undergo a saline suppression test because of cardiac illness; these patients were categorised as having incomplete investigations (“possible PA”). Seven people with positive screening results but normal ARR values on re‐testing were categorised as not having PA. [Correction added on 4 March 2022 after first online publication: the first sentence has been updated.]
Forty‐six of the 47 participants with two abnormal ARR values underwent confirmatory saline suppression testing; the PA diagnosis was confirmed for 35 people: 21 in recumbent tests, 14 in seated tests (14% of all eligible participants; 95% CI, 10–19%) (Box 1). In a sensitivity analysis excluding nine patients with incomplete investigations, the proportion of patients diagnosed with PA was 15% (95% CI, 10–20%). For the six GPs who recruited more than ten patients each, 10 of 116 participants had confirmed PA (9%), as did 25 of the 131 patients recruited by the other 64 GPs (19%). [Correction added on 4 March 2022 after first online publication: the first sentence has been updated.]
For the 62 people with positive screening results, the baseline characteristics — mean age, sex distribution, baseline blood pressure levels, and laboratory values (including serum potassium concentration) — of the three participant groups (confirmed PA, no PA, investigation incomplete) were similar (Box 2).
Of the 35 patients with confirmed PA, 16 underwent AVS subtyping; three were found to have unilateral PA (Box 1). Two of these patients underwent laparoscopic unilateral adrenalectomy with complete clinical and biochemical success; 6–12 months after surgery, their blood pressure was normal without the aid of antihypertensive medications, and their ARR had declined (in one case, from 82 pmol/mU to 15 pmol/mU; in the other, from 180 pmol/mU to 2 pmol/mU). The third patient had left‐side AVS lateralisation, but CT imaging also indicated a right adrenal lesion of indeterminate character that is now subject to surveillance, and they are being treated with a mineralocorticoid receptor antagonist.
The 13 patients with bilateral PA determined by AVS and the 12 who did not undergo AVS (chiefly because of advanced age and comorbid conditions) were managed medically. At their first consultations in the Endocrine Hypertension Clinic, 18 of these people were not taking antihypertensive medications, and seven had commenced one antihypertensive medication after initial ARR screening. By their final study visit to our clinic (median of 1.6 years [IQR, 1–2 years] since their referral), 18 of the 25 (72%) were taking spironolactone as their sole antihypertensive medication (median daily dose, 25 mg per day; IQR, 25–50 mg per day), three used spironolactone and a second antihypertensive agent, one had stopped using spironolactone because of side effects, and two had started using it too recently to tell whether they would need a second medication; one person was lost to follow‐up. For the 18 patients using spironolactone monotherapy, the median renin concentration increased from 3.7 mU/L (IQR, 2.2–5.3 mU/L) before treatment to 21.5 mU/L (IQR, 11.3–39.2 mU/L) after treatment; their median systolic blood pressure declined from 155 mmHg (IQR, 146–168 mmHg) to 122 mmHg (IQR, 120–129 mmHg), and their median diastolic blood pressure from 92 mmHg (IQR, 84–99 mmHg) to 85 mmHg (IQR, 75–88 mmHg).
Discussion
In this first study of PA screening in Australian primary care, we found that 35 of 247 treatment‐naïve people with hypertension had PA (14%). A similar value (16%) was recently reported in the United States, based on suppressed renin and high urinary aldosterone levels in 115 patients with stage 1 hypertension.26 In a survey of GPs, we had previously found that PA had been diagnosed in less than 0.1% of more than 7000 patients with hypertension.9 Our study suggests that PA is much more frequent in unselected general practice patients with hypertension than is recognised. Our finding also highlights the central role of GPs in the early detection of this treatable form of hypertension.
PA is considerably underdiagnosed in Australia. The BEACH (Bettering the Evaluation And Care of Health) dataset, with data for more than 15 000 GPs across Australia, included only 57 PA diagnoses for 1.5 million GP encounters over 16 years; aldosterone levels had been measured only 66 times.9 Even conservatively assuming a prevalence of 10% (the lower bound of the 95% CI for our estimate), a considerable number of people with undiagnosed PA could be missing targeted treatment. We found that a large majority of people diagnosed with PA benefited from targeted treatment; hypertension was resolved for two by adrenalectomy, and blood pressure control and normalisation of renin level was achieved by 18 of 25 patients prescribed mineralocorticoid receptor antagonist monotherapy.
People with PA benefit from early detection and diagnosis,4,5 but many have resistant hypertension and end‐organ damage by the time they are referred to a tertiary centre.8 The response to targeted PA treatment is inversely proportional to the duration of disease; that is, damage accumulates over time because of continuous mineralocorticoid receptor activation or chronic poor hypertension control.4,5 As early treatment is beneficial and early diagnosis enables early treatment, screening for PA is best initiated in general practice, early in the course of the disease. We found in our study that a dedicated education program could encourage GPs to actively screen for PA.
Another important finding was that the baseline characteristics of patients with and without PA were similar. PA is often assumed to chiefly affect younger patients and to be associated with hypokalaemia and severe hypertension. However, age, blood pressure, and serum potassium levels were similar in patients with PA or essential hypertension.
Limitations
The prospective design of our study and the active involvement of GPs in the screening process were strengths. It is important that screening was undertaken by the GPs rather than the investigators, as GPs are the clinicians most likely to diagnose and treat hypertension. The detection of PA in our study accordingly reflects what can be achieved by the targeted education of GPs. However, a limitation of our approach was the lack of information about the number of patients diagnosed with hypertension by participating GPs; if GPs screened patients selectively, selection bias may have ensued. Differences in selection for screening may have been related to patient‐, GP‐, or practice‐related factors, and we have commenced a follow‐up study to explore the barriers and enablers of PA screening from the GP perspective. Further, the patients in our study had predominantly presented to GPs in a large metropolitan city (Melbourne), and our findings may not be generalisable to rural, remote, or Indigenous Australian practices. As younger people may be less likely to see their GP than older people, they may not have been adequately sampled in our study.
Conclusion
We found that 14% of treatment‐naïve patients with hypertension screened in primary care had PA, a rate much higher than the current PA detection rate. Our findings suggest that it would be useful to evaluate the cost‐effectiveness of screening all patients with hypertension for PA in primary care before initiating antihypertensive treatment. If cost‐effective and acceptable to patients, PA screening could substantially change the management of hypertension in Australian primary care, and also bring GPs to the forefront of the timely detection and optimal management of a common disease.
Box 1 – Screening and confirmation of primary aldosteronism in our study
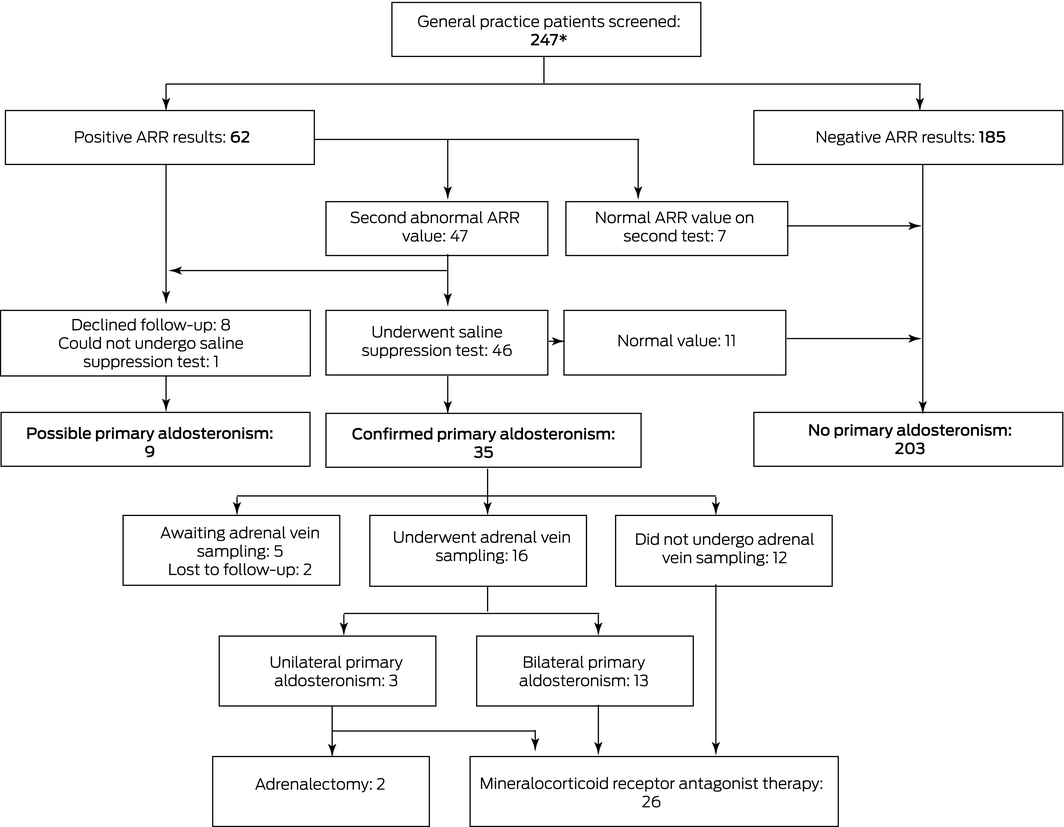
ARR = aldosterone‐to‐renin ratio. A positive screening test was defined by an ARR of 70 pmol/mU or more. * A further nine patients were screened, but were excluded from our analysis because they were not treatment‐naïve. [Correction added on 4 March 2022 after first online publication: figure has been updated.]
Box 2 – Characteristics of 247 treatment‐naïve general practice patients with hypertension screened for primary aldosteronism
|
|
All participants |
Participants with positive screening results |
|||||||||||||
|
Negative screen result |
Positive screen result |
P |
Primary aldosteronism confirmed |
Primary aldosteronism not confirmed |
Investigation incomplete |
P |
|||||||||
|
|
|||||||||||||||
|
Number of patients |
185 |
62 |
|
35 |
18 |
9 |
|
||||||||
|
Age (years), mean (SD) |
53 (14) |
55 (13) |
0.46 |
56 (11) |
50 (14) |
62 (13) |
0.06 |
||||||||
|
Sex (men) |
85 (46%) |
22 (36%) |
0.15 |
16 (46%) |
4 (22%) |
2 (22%) |
0.16 |
||||||||
|
Systolic blood pressure (mmHg), median (IQR) |
157 |
155 |
0.69 |
155 |
154 |
162 |
0.79 |
||||||||
|
Diastolic blood pressure (mmHg), median (IQR) |
94 |
95 |
0.80 |
93 |
95 |
92 |
0.55 |
||||||||
|
Laboratory values, median (IQR) |
|
|
|
|
|
|
|
||||||||
|
Potassium (mmol/L) |
4.4 |
4.3 |
0.19 |
4.3 |
4.4 |
4.4 |
0.17 |
||||||||
|
Creatinine (µmol/L) |
71 |
71 |
0.67 |
73 |
72 |
61 |
0.20 |
||||||||
|
eGFR (mL/min/1.73 m2) |
90 |
87 |
0.56 |
90 |
87 |
90 |
0.22 |
||||||||
|
Aldosterone (pmol/L) |
312 |
409 |
< 0.001 |
417 |
380 |
378 |
0.76 |
||||||||
|
Renin (mU/L) |
16.0 |
3.2 |
< 0.001 |
4.0 |
2.9 |
3.0 |
0.58 |
||||||||
|
ARR (pmol/mU) |
22 |
101 |
< 0.001 |
106 |
98 |
95 |
0.90 |
||||||||
|
|
|||||||||||||||
|
ARR = aldosterone‐to‐renin ratio; eGFR = estimated glomerular filtration rate; IQR = interquartile range; SD = standard deviation. Reference ranges: potassium, 3.5–5.2 mmol/L; creatinine, 45–90 µmol/L; eGFR, > 59 mL/min/1.73m2; plasma aldosterone, 70–1090 pmol/L; direct renin, 4.4–46.0 mU/L; ARR, < 70 pmol/mU. |
|||||||||||||||
Received 2 March 2021, accepted 10 September 2021
- Renata Libianto1,2
- Grant M Russell3
- Michael Stowasser4
- Stella M Gwini5
- Peta Nuttall2
- Jimmy Shen1,2
- Morag J Young2
- Peter J Fuller1,2
- Jun Yang1,3
- 1 Monash Health, Melbourne, VIC
- 2 Centre for Endocrinology and Metabolism, Hudson Institute of Medical Research, Melbourne, VIC
- 3 Monash University, Melbourne, VIC
- 4 Endocrine Hypertension Research Centre, University of Queensland, Brisbane, QLD
- 5 University Hospital Geelong, Geelong, VIC
This investigation was supported by a National Health and Medical Research Council (NHMRC) Ideas grant (APP1184927), the Monash Partners Medical Research Future Fund (MRFF) Rapid Applied Research Translation fund, a CASS Foundation grant, a Perpetual IMPACT grant (IPAP2019/1550), and a National Heart Foundation Vanguard grant. Renata Libianto was supported by an NHMRC/National Heart Foundation postgraduate scholarship and the Royal Australasian College of Physicians. Jun Yang was supported by project grants from the Endocrine Society of Australia, the National Heart Foundation, the CASS Foundation, and the High Blood Pressure Research Council Australia. Morag Young is supported by an Alice Baker and Eleanor Shaw Gender Equity Fellowship and by Baker Trustees. The Hudson Institute is supported by the Operational Infrastructure Scheme of the Victorian Government.
We thank all participating general practitioners and their practices for taking the time to screen patients for our study, including Edward Tsui, Jason Oh, and Terence Heng. We particularly thank Elise Forbes (Endocrine Hypertension team at Monash Health) for nursing and administrative support. Finally, we acknowledge the support of the Endocrine Hypertension team at Monash Health, including James Doery, Zhong Lu and Ken Wan (Department of Chemical Pathology); Winston Chong and Ken Lau (Department of Diagnostic Imaging); and James Lee and Simon Grodski (Department of Endocrine Surgery).
No relevant disclosures.
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment. An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 1889–1916.
- 2. Burrello J, Monticone S, Losano I, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension 2020; 75: 1025–1033.
- 3. Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2018; 6: 41–50.
- 4. Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 2008; 168: 80–85.
- 5. Williams TA, Lenders JWM, Mulatero P, et al; Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol 2017; 5: 689–699.
- 6. Libianto R, Fuller PJ, Young MJ, Yang J. Primary aldosteronism is a public health issue: challenges and opportunities. J Hum Hypertens 2020; 34: 478–486.
- 7. Rossi E, Perazzoli F, Negro A, Magnani A. Diagnostic rate of primary aldosteronism in Emilia‐Romagna, Northern Italy, during 16 years (2000–2015). J Hypertens 2017; 35: 1691–1697.
- 8. Lim YY, Shen J, Fuller PJ, Yang J. Current pattern of primary aldosteronism diagnosis: delayed and complicated. Aust J Gen Pract 2018; 47: 712–718.
- 9. Yang J, Fuller PJ, Stowasser M. Is it time to screen all patients with hypertension for primary aldosteronism? Med J Aust 2018; 209: 57–59. https://www.mja.com.au/journal/2018/209/2/it‐time‐screen‐all‐patients‐hypertension‐primary‐aldosteronism
- 10. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol 2017; 69: 1811–1820.
- 11. Galati SJ, Cheesman KC, Springer‐Miller R, et al. Prevelence of primary aldosteronism in an urban hypertensive population. Endocr Pract 2016; 22: 1296–1302.
- 12. Käyser SC, Deinum J, de Grauw WJC, et al. Prevalence of primary aldosteronism in primary care: a cross‐sectional study. Br J Gen Pract 2018; 68: e114–e122.
- 13. Volpe C, Wahrenberg H, Hamberger B, Thorén M. Screening for primary aldosteronism in a primary care unit. J Renin Angiotensin Aldosterone Syst 2013; 14: 212–219.
- 14. Westerdahl C, Bergenfelz A, Isaksson A, et al. Primary aldosteronism among newly diagnosed and untreated hypertensive patients in a Swedish primary care area. Scand J Prim Health Care 2011; 29: 57–62.
- 15. Ito Y, Takeda R, Karashima S, et al. Prevalence of primary aldosteronism among prehypertensive and stage 1 hypertensive subjects. Hypertens Res 2011; 34: 98–102.
- 16. Fogari R, Preti P, Zoppi A, et al. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res 2007; 30: 111–117.
- 17. Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens 2006; 20: 129–136.
- 18. Westerdahl C, Bergenfelz A, Isaksson A, et al. High frequency of primary hyperaldosteronism among hypertensive patients from a primary care area in Sweden. Scand J Prim Health Care 2006; 24: 154–159.
- 19. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem 2005; 51: 386–394.
- 20. Xu Z, Yang J, Hu J, et al; Chongqing Primary Aldosteronism Study (CONPASS) group. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol 2020; 75: 1913–1922.
- 21. Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta‐regression analysis. J Clin Endocrinol Metab 2016; 101: 2826–2835.
- 22. Perschel FH, Schemer R, Seiler L, et al. Rapid screening test for primary hyperaldosteronism: ratio of plasma aldosterone to renin concentration determined by fully automated chemiluminescence immunoassays. Clin Chem 2004; 50: 1650–1655.
- 23. Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab 2018; 103: 4113–4124.
- 24. Chee NYN, Abdul‐Wahab A, Libianto R, et al. Utility of adrenocorticotropic hormone in adrenal vein sampling despite the occurrence of discordant lateralization. Clin Endocrinol (Oxf) 2020; 93: 394–403.
- 25. Hundemer GL, Curhan GC, Yozamp N, et al. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol 2018; 6: 51–59.
- 26. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism. Ann Intern Med 2020; 173: 10–20.





Abstract
Objective: To assess the identification of primary aldosteronism (PA) in newly diagnosed, treatment‐naïve patients with hypertension by screening in primary care.
Design: Prospective study.
Setting: General practices in the South Eastern Melbourne Primary Health Network with at least three general practitioners and general practices elsewhere in Victoria that referred patients to the Endocrine Hypertension Clinic at Monash Health, 2017‒2020.
Participants: Adults (18–80 years) with newly diagnosed hypertension (measurements of systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg on at least two occasions) and not taking antihypertensive medications were screened for PA by assessing their aldosterone‐to‐renin ratio (ARR). Participants with two ARR values exceeding 70 pmol/mU underwent saline suppression testing at the Endocrine Hypertension Service (Monash Health) to confirm the diagnosis of PA.
Main outcome measures: Prevalence of PA (number of patients with confirmed PA divided by number screened).
Results: Sixty‐two of 247 screened participants had elevated ARR values on screening (25%); for 35 people (14%; 95% CI, 10–19%), PA was confirmed by saline suppression testing. Baseline characteristics (mean age, sex distribution, median baseline blood pressure levels, and serum potassium concentration) were similar for people with or without PA.
Conclusion: PA was diagnosed in 14% of patients with newly diagnosed hypertension screened by GPs, indicating a potential role for GPs in the early detection of an important form of secondary hypertension for which specific therapies are available.