By November 2021, the global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) had infected over 251 million people worldwide and led to over 5 million deaths, with many countries experiencing second and third waves (or more) of infection.1 Australia’s response has included implementation of a range of public health measures to control the spread of SARS‐CoV‐2, including strict quarantine measures, reduction in international arrivals to Australia, isolation of cases, lockdowns, and contact tracing. Australia has experienced lower rates of infection and mortality than many other countries, with 185 627 confirmed SARS‐CoV‐2 infections and 1862 deaths (as of 11 November 2021).2 Older people experience higher rates of critical illness or death associated with coronavirus disease 2019 (COVID‐19), the disease caused by infection with SARS‐CoV‐2. In Australia, of the 3060 people diagnosed with SARS‐CoV‐2 infection who lived in residential aged care facilities, 812 died.3 For some people who become critically ill with COVID‐19, a decision may be made to take a palliative approach to their care, or they may be already receiving palliative care due to other pre‐existing illnesses.
The National COVID‐19 Clinical Evidence Taskforce
The National COVID‐19 Clinical Evidence Taskforce (the Taskforce) was established in March 2020 to give reliable, up‐to‐date advice to clinicians providing frontline care during the COVID‐19 pandemic. The Taskforce is comprised of 32 peak health professional bodies across Australia whose members provide clinical care to people with COVID‐19, including the Australian Association of Gerontology, the Australian COVID‐19 Palliative Care Working Group and the Australian and New Zealand Society for Geriatric Medicine. The Taskforce is managed in partnership with Cochrane Australia and the Australian Living Evidence Consortium. Systematic reviewers and guideline methodologists are charged with continually identifying, evaluating and synthesising the new research evidence. The evidence is then reviewed by one of the eight panels of clinical experts following rigorous, transparent methods based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to develop evidence‐based guidance. The guidance follows a living guideline approach, which means that it is updated continually as new evidence emerges.4 Living guideline outputs are approved by the Guideline Leadership Group and endorsed by the Steering Committee, which consists of representatives from the member professional bodies. The guideline recommendations are also reviewed by a Consumer Panel, convened in collaboration with the Consumers Health Forum of Australia to provide consumer input on preferences and values.
The Taskforce established a specialist panel to address the care of older people and those requiring palliative care (Box 1). The panel is comprised of a range of experts from geriatric medicine, palliative care, pharmacy, respiratory medicine, and occupational therapy, and includes representation from multiple Australian states and territories (New South Wales, Victoria, Queensland, South Australia, Western Australia, the Northern Territory, the Australian Capital Territory), and urban, rural and remote areas. The panel first convened on 2 June 2020 and has since met more than 20 times. This article describes the Taskforce guidance for the care of older people living with frailty or cognitive impairment and people needing palliative care with COVID‐19.
Methods
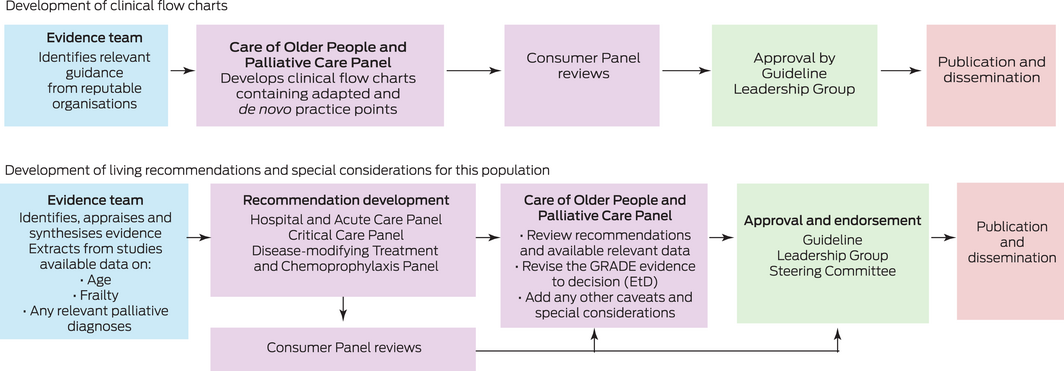
The Care of Older People and Palliative Care Panel was tasked with developing clinical flow charts to address key topics not currently covered in the living guideline recommendations. The Panel was also tasked with reviewing the living guideline recommendations relevant to this population and developing any population‐specific guidance where appropriate.
Methods for developing clinical flow charts
The Care of Older People and Palliative Care Panel discussed the need for clinical advice for these specific patient groups and decided that clinical flow charts would be the best way to present this advice.
To develop these clinical flow charts, we conducted web searches of national and international organisations and professional societies, as well as Australian state and territory health departments. We identified relevant publicly available guidelines with recommendations relating to the clinical care of older people living with frailty or cognitive impairment and people with advanced life‐limiting diseases requiring palliative care with suspected or confirmed COVID‐19. The existing guidance was discussed by the Panel. Practice points were adapted from Australian and international guidelines and de novo practice points were developed. The flow charts are reviewed by the panel and updated as required (Box 2).
Methods for developing living guideline recommendations
The Taskforce uses living guideline methods to produce guideline recommendations, whereby relevant new evidence is identified through continual literature surveillance and rapidly incorporated into updated guidelines, maintaining the currency of guideline recommendations in near real‐time. Living guideline approaches have been applied in rapidly moving clinical areas, such as stroke and diabetes, but the unprecedented speed and volume of evidence publication during the COVID‐19 pandemic has posed new challenges.5,6,7 The Taskforce has responded by producing weekly updates of their living guidance, the most rapid update cycle of which we are aware.4
The Taskforce guidelines were developed to meet the Australian National Health and Medical Research Council (NHMRC) standards and using GRADE methods. The Taskforce uses the MAGIC (Making GRADE the Irresistible Choice) digital platform (https://magicevidence.org/) and its implementation of the Evidence to Decision (EtD) framework.8 The living recommendations presented in this article were approved by the NHMRC on 15 February 2021. The Taskforce’s living guideline methodology is published4 and available at https://covid19evidence.net.au/more‐about‐the‐guidelines/.
The Taskforce has developed 129 recommendations for the clinical care of people with COVID‐19. Each of these recommendations was developed by one of the Taskforce panels: Primary and Chronic Care Panel, Hospital and Acute Care Panel, Critical Care Panel, Disease‐Modifying Treatment and Chemoprophylaxis Panel, Pregnancy and Perinatal Care Panel, and Paediatric and Adolescent Care Panel. Where available, data such as age, frailty and relevant palliative diagnoses are extracted from the studies by the evidence team using the Covidence systematic review software (www.covidence.org). The Care of Older People and Palliative Care Panel review relevant adult living recommendations for any changes pertinent to these populations, focusing on recommendations developed for disease‐modifying treatments and respiratory support. The process for developing living guideline recommendations is shown in Box 2.
Recommendations
The recommendations for the care of older people and those who require palliative care with COVID‐19 consist of two clinical flow charts (online Supporting Information) and additional considerations for the adult living guideline recommendations (Box 3, Box 4 and Box 5). The clinical flow charts provide a scaffolding for all areas of care pertinent to these populations, including reference to the living guideline recommendations. The rationale for the recommendations and additional considerations for older people and those who require palliative care with COVID‐19 are discussed below.
Clinical flow charts for the care of older people and palliative care
The Care of Older People and Palliative Care Panel developed two clinical flow charts:
- ▪ management of people with COVID‐19 who are older and living with frailty and/or cognitive impairment; and
- ▪ management of people with COVID‐19 who are receiving palliative care.
The two flow charts have overlapping principles, but each emphasises aspects of care pertinent to the specified population. The clinical flow charts (online Supporting Information) incorporate the Taskforce’s living guideline recommendations, endorsed guidance and practice points. The flow charts also refer to guidance from the Australian and New Zealand Society of Palliative Medicine for further advice on specific treatment issues, such as the use of benzodiazepines.9
The flow charts cover key areas of clinical care: goals of care, communication, medication management, active disease‐directed care, key clinical and symptom management issues (delirium, anxiety and agitation, breathlessness or cough) and escalation of care decisions. The practice points provide guidance on the delivery of quality geriatric and palliative care, underpinned by a commitment to individualising treatment to the person’s goals of care, acknowledgement of caregivers, and with guidance on modified approaches that can address challenges to these areas of care during the COVID‐19 pandemic.
Respiratory support (living guideline recommendations, section 8)
The Critical Care Panel and the Hospital and Acute Care Panel have developed 13 living recommendations for respiratory support interventions that should be offered or considered for some adult patients with COVID‐19. Box 3 shows the adult living recommendations (guideline version 37.0 on 6 April 2021) with additional considerations developed by the Care of Older People and Palliative Care Panel pertinent to these populations. Respiratory support options for those with frailty or underlying health issues may differ or be limited, and this required additional population guidance to supplement the general adult respiratory support recommendations. These include non‐invasive interventions, such as prone positioning, and more invasive forms of therapy, including intubation and mechanical ventilation, and rescue therapies, such as extracorporeal membrane oxygenation. When deciding whether to proceed with a therapy, the net clinical benefit for each patient should be considered on a case‐by‐case basis, as factors such as frailty, advanced illness, or comorbidity may lessen the benefit and increase potential harms. In particular, some older people living with frailty may be at increased risk of harm, and the nature of the clinical benefits (eg, symptom benefits) may be limited or unclear for them.
Disease‐modifying treatments (living guideline recommendations, section 6)
The Disease‐Modifying Treatment and Chemoprophylaxis Panel has recommended the use of corticosteroids, and conditionally recommended the use of remdesivir and tocilizumab, in certain patients with COVID‐19. Box 4 shows the adult living recommendations (guideline version 37.0 on 6 April 2021) with additional considerations developed by the Care of Older People and Palliative Care Panel pertinent to these populations. The identified trials on disease‐modifying treatments lacked available data on frailty, cognitive impairment and relevant diagnoses that may indicate a patient was receiving palliative care. While people aged over 65 years were included in many trials, most patients recruited were less than 65 years of age. After reviewing the relevant adult living recommendations, the Care of Older People and Palliative Care Panel adopted the approach of drawing inferences from the overall trial evidence, applying these inferences to older and palliative care populations, and adopting the adult recommendations until further evidence in these populations is available.10
Disease‐modifying treatments not recommended outside the context of randomised controlled trials with appropriate ethics approval (living guideline recommendations, section 6)
The Disease‐Modifying Treatment and Chemoprophylaxis Panel has developed 42 recommendations (guideline version 37.0 on 6 April 2021) for treatments that should not be used outside the context of randomised controlled trials with appropriate ethics approval. Box 5 shows the adult living recommendations (guideline version 37.0 on 6 April 2021) with additional considerations for trial design and inclusions pertinent to older people living with frailty or cognitive impairment and those receiving palliative care. There remains uncertainty in the evidence for these treatments, and until further evidence is available, they should not be used in practice. Therefore, older people living with frailty or cognitive impairment and those receiving palliative care should only receive these treatments if they are eligible to be enrolled in randomised trials.
Discussion
The clinical flow charts are available online at https://covid19evidence.net.au/#clinical‐flowcharts. The living recommendations on the clinical care of people with COVID‐19 with additional considerations, where appropriate, on the care of older people and those requiring palliative care are available at https://covid19evidence.net.au/.
Older people living with frailty or cognitive impairment and people with advanced life‐limiting illnesses needing palliative care may be at risk of poorer outcomes and more serious illness, associated with multiple underlying clinical conditions including COVID‐19. The flow charts developed by the Care of Older People and Palliative Care Panel of the National COVID‐19 Clinical Evidence Taskforce aim to address the key challenges to delivering patient care resulting from COVID‐19 in these populations.
Along with treatment decisions, other elements of clinical care are particularly complex in these populations. Effective communication with patients can be hindered due to the physical barriers created by mask wearing and other forms of personal protective equipment. COVID‐19 also limits face‐to‐face contact, not just between the patient and health professional but also between the patient and their families and carers, due to visiting restrictions. The clinical flow charts encourage the use of alternative tools, such as digital technologies, which can be used safely from outside the patient’s room and without requiring personal protective equipment. This ensures adequate communication with patients that supports the development of trusting therapeutic relationships between patients and health professionals.
Another important challenge relates to advance care plans or directives. Individuals who had advance care plans or directives likely wrote them before the COVID‐19 pandemic. However, the situations experienced throughout the COVID‐19 pandemic may have influenced individuals’ preference regarding treatments in ways that were not captured by these documents previously. This issue stresses the importance of advance care planning being an active, iterative process that develops over time with ongoing discussions regarding patients’ goals of care. It is vital to reaffirm these wishes with patients and any legal guardian for medical decision making. For patients with COVID‐19 and other underlying health conditions, their goal of care may be curative, directed towards COVID‐19, or it may focused instead on symptom management. Care should always be holistic, respecting the priorities and preferences of patients.
There is uncertainty around trial evidence for older people living with frailty or cognitive impairment and those requiring palliative care with COVID‐19. Historically, these populations have not been included in trials and this trend has continued with COVID‐19 trials. When trials have included these populations, they have often lacked available data on frailty, cognitive impairment, and relevant advanced life‐limiting comorbidities that may indicate the person was receiving a palliative approach to care. The safety and effectiveness of many treatments are therefore more uncertain in these patient populations; however, the Panel advised that most recommendations within the living guidelines should still apply, unless contraindicated.
As people in these populations are particularly at risk from COVID‐19, the Care of Older People and Palliative Care Panel encourages the conduct of trials that include older people living with frailty and those receiving palliative care. These trials should also record appropriate baseline measurements of frailty, cognitive impairment, and any relevant advanced comorbidities indicating a palliative approach to care.11 In addition, for trials in people requiring palliative care, their outcomes should consider symptom management and quality of life.
Conclusion
Our Panel has developed clinical flow charts to provide crucial advice to health professionals working with older people and people requiring palliative care with COVID‐19 in Australia (https://covid19evidence.net.au/#clinical‐flowcharts), and thus provide a global resource, accessed by countries across the world. The Taskforce has developed 129 recommendations for people infected with SARS‐CoV‐2 and has included special considerations, where appropriate, on the care of older people and those requiring palliative care (https://covid19evidence.net.au/). The guidance was achieved through a unique collaboration among health professionals across Australia, providing wide ranging views and experience to produce robust and trustworthy guidance. We encourage regular visits to our website, given the rapidly changing nature of evidence in the COVID‐19 pandemic and the Taskforce’s commitment to providing timely, up‐to‐date, living guidance.
Box 1 – Population definitions
|
|
|||||||||||||||
|
Older people with frailty or cognitive impairment and COVID‐19:
|
|||||||||||||||
|
People with COVID‐19 and who require palliative care:
|
|||||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019. |
|||||||||||||||
Box 3 – Respiratory support recommendations (guideline version 37.0 on 6 April 2021, section 8)
|
|
|||||||||||||||
|
Recommendations with additional considerations added for older people and those needing palliative care*
|
|||||||||||||||
|
Special considerations for older people and those needing palliative care |
|||||||||||||||
|
|||||||||||||||
|
Recommendations without additional considerations added for older people and those needing palliative care* |
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; COVID‐19 = coronavirus disease 2019; ECMO = extracorporeal membrane oxygenation; HFNO = high flow nasal oxygen; NIV = non‐invasive ventilation; PEEP = positive end‐expiratory pressure; PPE = personal protective equipment. |
|||||||||||||||
Box 4 – Disease modifying treatments recommended for the treatment of coronavirus disease 2019 (COVID‐19; guideline version 37.0, 6 April 2021, section 6)
|
|
|||||||||||||||
|
Dexamethasone* |
|
||||||||||||||
|
Remdesivir* |
|
||||||||||||||
|
Special considerations for older people living with frailty and those receiving palliative care |
It is unclear whether older people or those requiring palliative care were included in the studies on which these recommendations are based. Until further evidence in these populations is available, the Taskforce does not believe a different recommendation should apply, unless contraindicated. Additional variability may be expected in these populations given the potentially different preferences and values placed on outcomes and goals for care, such as symptom relief. Because the benefit to harm ratio is uncertain, the acceptability may vary in these populations due to individual decision making around goals of care. |
||||||||||||||
|
|
|||||||||||||||
|
* The primary panel for the recommendations is the Disease‐Modifying Treatment and Chemoprophylaxis Panel. Recommendations are reviewed by the Guidelines Leadership Group and approved by the Steering Committee before being published. In addition, all recommendations are reviewed by the Consumer Panel. |
|||||||||||||||
Box 5 – Disease‐modifying treatments not recommended for the treatment of coronavirus disease 2019 (COVID‐19; guideline version 37.0, 6 April 2021, section 6)
|
|
|||||||||||||||
|
Do not use the following for the treatment of COVID‐19*: |
|||||||||||||||
|
|
|
|||||||||||||
|
Do not use the following disease modifying treatments for the treatment of COVID‐19 outside of randomised trials with appropriate ethics approval:* |
|||||||||||||||
|
|
|
|||||||||||||
|
Special considerations for older people living with frailty and those receiving palliative care: |
|||||||||||||||
|
Trials are needed in special populations, including older people living with frailty and those receiving palliative care, for treatments currently recommended only in the context of randomised controlled trials with appropriate ethics approval. Until further evidence is available, do not use these disease‐modifying treatments to treat COVID‐19 in these populations unless they are eligible to be enrolled in trials. As older people living with frailty or cognitive impairment are particularly at risk from COVID‐19, we encourage trials that include this population (with appropriate baseline measurement of frailty and cognitive impairment). In people requiring palliative care, trials should consider symptom management and quality of life outcomes. Because the benefit to harm ratio is uncertain, acceptability may vary due to individual decision making around goals of care. Given the absence of trials and uncertain benefit to harm ratio, this recommendation protects these more vulnerable populations. |
|||||||||||||||
|
|
|||||||||||||||
|
* The primary panel for the recommendations is the Disease‐Modifying Treatment and Chemoprophylaxis Panel. Recommendations are reviewed by the Guidelines Leadership Group and approved by the Steering Committee before being published. In addition, all are reviewed by the Consumer Panel. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Saskia Cheyne1,2
- Richard I Lindley3,4
- Natasha Smallwood5,6
- Britta Tendal2
- Michael Chapman7
- David Fraile Navarro8
- Phillip D Good9
- Peter Jenkin10
- Steve McDonald2
- Deidre Morgan11,12
- Melissa Murano2
- Tanya Millard2
- Vasi Naganathan13
- Velandai Srikanth6
- Penelope Tuffin14,15
- Joshua Vogel2,16
- Heath White2
- Samantha P Chakraborty6
- Elizabeth Whiting17
- Leeroy William2,18
- Patsy M Yates19
- Mandy Callary20
- Julian Elliott2
- Meera R Agar21
- for the National COVID‐19 Clinical Evidence Taskforce
- 1 NHMRC Clinical Trials Centre, University of Sydney, Sydney, NSW
- 2 Cochrane Australia, Monash University, Melbourne, VIC
- 3 Westmead Applied Research Centre, University of Sydney, Sydney, NSW
- 4 George Institute for Global Health, Sydney, NSW
- 5 Alfred Hospital, Melbourne, VIC
- 6 Monash University, Melbourne, VIC
- 7 Canberra Hospital, Canberra, ACT
- 8 Australian Institute of Health Innovation, Macquarie University, Sydney, NSW
- 9 St Vincent’s Private Hospital, Brisbane, Brisbane, QLD
- 10 Resthaven, Adelaide, SA
- 11 Research Centre for Palliative Care, Death and Dying, Flinders University, Adelaide, SA
- 12 Flinders University, Adelaide, SA
- 13 Centre for Education and Research on Ageing (CRGH), University of Sydney, Sydney, NSW
- 14 Royal Perth Hospital, Perth, WA
- 15 Fiona Stanley Hospital, Perth, WA
- 16 Maternal, Child and Adolescent Health Program, Burnet Institute, Melbourne, VIC
- 17 Prince Charles Hospital, Brisbane, QLD
- 18 Eastern Health, Melbourne, VIC
- 19 Centre for Cancer and Palliative Care Outcomes, Queensland University of Technology, Brisbane, QLD
- 20 Royal Adelaide Hospital, Adelaide, SA
- 21 IMPACCT Centre, University of Technology Sydney, Sydney, NSW
We acknowledge all members of the National COVID‐19 Clinical Evidence Taskforce, the member organisations, partners, governments and funders that support the initiative (online Supporting Information). These guidelines have received funding from the Australian Government Department of Health; the Victorian Government Department of Health and Human Services; the Ian Potter Foundation; the Walter Cottman Endowment Fund, managed by Equity Trustees; and the Lord Mayor’s Charitable Foundation.
No relevant disclosures.
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis 2020; 20: 533–534.
- 2. Department of Health. Coronavirus (COVID‐19) case numbers and statistics [website]. https://www.health.gov.au/news/health‐alerts/novel‐coronavirus‐2019‐ncov‐health‐alert/coronavirus‐covid‐19‐current‐situation‐and‐case‐numbers#at‐a‐glance (viewed Mar 2021).
- 3. Department of Health. COVID‐19 outbreaks in Australian residential aged care facilities — 26 March 2021. https://www.health.gov.au/resources/collections/covid‐19‐outbreaks‐in‐australian‐residential‐aged‐care‐facilities (viewed Mar 2021).
- 4. Tendal B, Vogel JP, McDonald S, et al. Weekly updates of national living evidence‐based guidelines: methods for the Australian living guidelines for care of people with COVID‐19. J Clin Epidemiol 2021; 131: 11–21.
- 5. White H, Tendal B, Elliott J, et al. Breathing life into Australian diabetes clinical guidelines. Med J Aust 2020; 212: 250–251. https://www.mja.com.au/journal/2020/212/6/breathing‐life‐australian‐diabetes‐clinical‐guidelines
- 6. English C, Bayley M, Hill K, et al. Bringing stroke clinical guidelines to life. Int J Stroke 2019; 14: 337–339.
- 7. National Library of Medicine. LitCovid [website]. Bethesda, MD: NIH NLM, 2020. https://www.ncbi.nlm.nih.gov/research/coronavirus/faq (viewed Mar 2021).
- 8. Alonso‐Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016; 353: i2016.
- 9. Allcroft P, Blackburn P, Borbasi J, et al. ANZSPM Guidance — palliative care in the COVID‐19 context 2020. Canberra: Australia and New Zealand Society of Palliative Medicine, 2021. https://www.anzspm.org.au/c/anzspm?a=da&did=1005077&pid=1587788101 (viewed Mar 2021).
- 10. Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet 2001; 357: 373–380.
- 11. Lindley RI. Frailty should now be measured in all randomized controlled trials including older people. J Hypertens 2021; 39: 419–420.






Abstract
Introduction: Older people living with frailty and/or cognitive impairment who have coronavirus disease 2019 (COVID‐19) experience higher rates of critical illness. There are also people who become critically ill with COVID‐19 for whom a decision is made to take a palliative approach to their care. The need for clinical guidance in these two populations resulted in the formation of the Care of Older People and Palliative Care Panel of the National COVID‐19 Clinical Evidence Taskforce in June 2020. This specialist panel consists of nursing, medical, pharmacy and allied health experts in geriatrics and palliative care from across Australia.
Main recommendations: The panel was tasked with developing two clinical flow charts for the management of people with COVID‐19 who are i) older and living with frailty and/or cognitive impairment, and ii) receiving palliative care for COVID‐19 or other underlying illnesses. The flow charts focus on goals of care, communication, medication management, escalation of care, active disease‐directed care, and managing symptoms such as delirium, anxiety, agitation, breathlessness or cough. The Taskforce also developed living guideline recommendations for the care of adults with COVID‐19, including a commentary to discuss special considerations when caring for older people and those requiring palliative care.
Changes in management as result of the guideline: The practice points in the flow charts emphasise quality clinical care, with a focus on addressing the most important challenges when caring for older individuals and people with COVID‐19 requiring palliative care. The adult recommendations contain additional considerations for the care of older people and those requiring palliative care.