Abstract
Objectives: To review evidence for the increased incidence of late diagnosed developmental dysplasia of the hip (DDH) in South Australia; to identify perinatal risk factors associated with late DDH in babies born between 2003 and 2009 in SA.
Design: Linkage study of data collected prospectively by the South Australian Birth Defects Register (SABDR) and the Pregnancy Outcome Statistics Unit (SA Department of Health), supplemented by medical records review.
Participants: All children born 2003–2009 in whom DDH was diagnosed between 3 months and 5 years of age and notified to the SABDR (data inclusion range, 2003–2014). Children with teratological hip dislocations and other major congenital abnormalities were excluded.
Main outcome measures: Uni- and multivariable analyses were performed to identify perinatal risk factors for late diagnosed DDH.
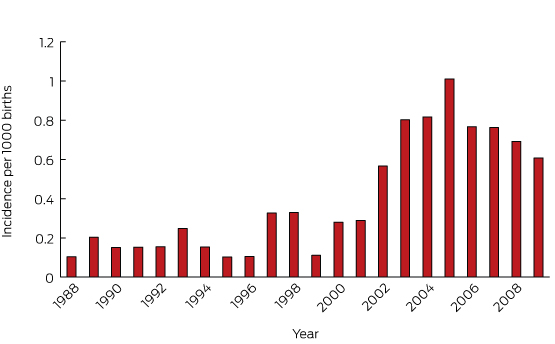
Results: The incidence of late diagnosed DDH in babies born 2003–2009 was 0.77 per 1000 live births, contrasting with the figure of 0.22 per 1000 live births during 1988–2003. Significant perinatal risk factors were birth in a rural hospital (v metropolitan public hospital: odds ratio [OR], 2.47; CI, 1.37–4.46; P = 0.003), and being the second child (v being the first-born: OR, 1.69; CI, 1.08–2.66; P = 0.023). Breech presentation was highly significant as a protective factor when compared with cephalic presentation (OR, 0.25; CI, 0.12–0.54; P < 0.001).
Conclusions: The incidence of late DDH has increased in SA despite an ongoing clinical screening program. Increased awareness, education, and avoidance of inappropriate lower limb swaddling are necessary to reverse this trend.
Early detection and treatment of developmental dysplasia of the hip (DDH) in newborns is important because late diagnosis (later than 3 months of age) is associated with a significant risk of poorer outcomes. This includes increased likelihood of surgery, more invasive surgical procedures, longer hospital stays, and early osteoarthritis of the hip, as well as increased health care costs.1,2
We previously reported a worrying increase in the number of infants diagnosed between 3 and 18 months of age with DDH in South Australia; prospective data showed an incidence of late diagnosed DDH of greater than 0.7 cases per 1000 live births, or around 15 cases each year.3 This contrasts with the low rate of 0.22 per 1000 live births, or four to five cases per year, during the period 1988–2003,4 and has occurred despite continued routine physical examination screening of all neonatal hips. Reports from New South Wales5 and Western Australia6 confirm that the increased incidence of late diagnosed DDH is not limited to SA, but has been observed nationally.
The aim of our study was to determine whether there are identifiable perinatal risk factors associated with late DDH in babies born in SA since 2003, and to review the treatment required for patients with late DDH. Additionally, we provide a discussion of current screening practices and discuss strategies to redress the increased incidence of late DDH in Australia.
Methods
The South Australian Birth Defects Register (SABDR) has received notifications since 1986 of all cases of DDH diagnosed during the first 5 years of life. Notifications are received from a wide range of health professionals and treatment facilities, and are mandatory under the SA Health Care Act.
All diagnoses of DDH in children born in SA from 2003 to 2009 and notified to SABDR were identified for this study. Notifications for a given birth year are not considered complete until all children have achieved their fifth birthday, allowing for a late diagnosis. Consequently, all patients in our series were followed up for a minimum of 5 years after birth, with data collection ceasing at the end of 2014.
Late DDH was defined as an initial diagnosis of DDH at or after 3 months of age. Our methods were identical with those of a published SA study that reported data from the same institution for 1988–2003,4 and there were no changes in reporting practices. Incidence data from the earlier and current studies were combined to produce an incidence graph of late DDH for 1988–2009.
Cases were linked with the Pregnancy Outcome Statistics Unit of the SA Department of Health, which also collects details on mother and baby as a legislative requirement. These data include congenital abnormalities diagnosed at birth, as well as socio-demographic and clinical information.
Teratological hip dislocations and patients with an SABDR notification of significant congenital or genetic anomaly at any time were excluded.
Statistical methods
Likelihood ratio χ2 tests, Fisher exact tests, and logistic regression analysis were used to examine possible significant predictors at the univariable level; predictors with a trend to significance (P < 0.1) were then included in a multivariable logistic regression model, using backward selection. Statistical significance was defined as P < 0.05.
Ethics approval
Approval for the study protocol was granted by the Women’s and Children’s Health Network Human Research Ethics Committee (HREC/13/WCHN/68).
Results
Among babies born between 2003 and 2009, 902 cases of DDH were notified in SA. Twenty-four cases were excluded from our analysis; the timing of the diagnosis in five patients could not be confirmed, and in 19 cases a significant genetic disorder was subsequently diagnosed. Of the remaining 878 children, 777 were diagnosed before 3 months of age, and 101 children were diagnosed at or after 3 months of age. The incidence of late DDH in babies born between 2003 and 2009 was therefore 0.77 per 1000 live births; late diagnosed DDH represented 11.5% of all cases of DDH.
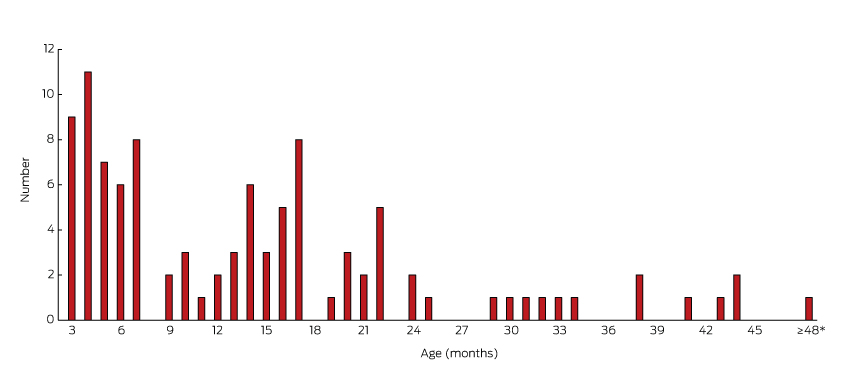
The incidence of late DDH by year of birth ranged between 0.6 and 1.0 per 1000 live births during 2003–2009. Box 1 combines the current data with previously published SA data to show the trend in late diagnosed DDH cases in babies born from 1988 to 2009. The oldest child was diagnosed at 4 years and 5 months of age; 52% of late diagnosed children were over 12 months of age (Box 2).
The results of univariable and multivariable analysis of risk factors associated with late DDH are summarised in Box 3. Significant factors at the univariable level were rural birth (odds ratio [OR], 2.65; 95% confidence interval (CI), 1.49–4.70; P = 0.001), being the second child (v being the first-born: OR, 2.05; CI, 1.33–3.17; P = 0.001), and female sex (OR, 2.06; CI, 1.07–3.94; P = 0.03). Breech presentation was protective against late diagnosis DDH (OR, 0.22; CI, 0.10–0.45; P < 0.001).
Significant perinatal risk factors (multivariable analysis) were birth in a rural hospital (v metropolitan public hospital: OR, 2.47; CI, 1.37–4.46; P = 0.003), and being the second child (v being the first-born: OR, 1.69; CI, 1.08; P = 0.023). Breech presentation was highly significant as a protective factor when compared with cephalic presentation (OR, 0.25; CI, 0.12–0.54; P < 0.001).
There was a trend to a shorter hospital stay for late DDH than for children with an early diagnosis of DDH (P = 0.075), and a significantly shorter hospital stay when compared with the entire population of live births (P = 0.038).
There was no association between either maternal age or maternal ethnic group and late DDH; 91% of babies with late DDH and 93% of babies with early DDH were born to mothers of European descent.
Thirty-one of the 101 children diagnosed with late DDH were successfully treated with splints alone. A further 25 had a successful closed reduction under general anaesthesia, with or without percutaneous adductor tenotomy, between 3.5 and 22 months (mean age, 9.7 months; standard deviation, 5.1 months). An open surgical procedure was required for 44 children. Seven patients required a second open reduction to achieve a reduced hip, and two patients underwent a third open reduction. Thirty-seven pelvic and 15 femoral osteotomies were performed.
Discussion
The incidence of DDH diagnosed at or after 3 months of age in babies born in SA between 2003 and 2009 was 0.77 per 1000 live births, comprising 11.5% of all DDH diagnoses. This has increased from 0.22 per 1000 live births, or 3.5% of all DDH cases, for babies born 1988–2003.4 There has been only a minor increase in the overall incidence of DDH, from 6.4 per 1000 live births for 1988–2003 to 6.8 per 1000 live births for 2003–2009.
Fifty-two of the 101 late DDH cases born between 2003 and 2009 were children of walking age. This compares with NSW data showing a tenfold increase in hip dislocations diagnosed in walking age children5 and a WA report of 17 patients diagnosed between the ages of 6 months and 5 years during 2010.6
Late diagnosed DDH is considered a significant public health issue because late diagnosis and treatment is associated with a lower likelihood of success, higher rates of surgery, increased complications (such as avascular necrosis of the femoral head1,2), and increased health care costs, including those associated with hospital admission, general anaesthesia, and the fact that 44% of such patients require major operative procedures.
The increased incidence of late DDH in Australia has occurred despite an ongoing clinical screening program that involves physical examination of all babies at birth, at 6 weeks, and at 3, 6 and 12 months of age, as well as increasing use of selective ultrasound screening.4,5 This approach has been used for patients with known risk factors for DDH, such as breech presentation and a positive family history, and may be used where the clinical examination is inconclusive.4,7 The American Academy of Pediatrics recommends routine ultrasound screening at 6 weeks of age for girls born in the breech position, with optional screening for breech boys, as well as for girls with a family history of DDH.7 Our results support previous findings8 that selective ultrasound screening fails to prevent the occurrence of late presenting DDH patients, as the majority of babies requiring intervention are not identified by the current criteria.
It is noteworthy that breech presentation was protective, a finding that corroborates previous SA data.4 One could speculate that this known risk factor for DDH increases the physician’s awareness of the potential for dysplasia and promotes extra care when examining the baby. Another risk factor for late DDH was being the second child born to a mother. Clinicians should remain mindful that DDH can occur in any child, and late presentations occur in children without traditional risk factors.
An increase in the number of late DDH diagnoses has also been observed in the United Kingdom, North America and France;8-11 it has been attributed to external factors, such as swaddling, as well as to reduced clinical expertise in hip examination, and the lack of repeated hip checks of children until walking age.8
A systematic review has cast doubt on the value of screening for DDH in general, citing a lack of evidence for benefits in long term functional outcomes, as well as the risk of overtreating.12 This highlights the challenges when making recommendations for screening, with differences in definitions, treatment protocols and outcome measures making conclusions difficult. DDH encompasses a broad spectrum of conditions, including neonatal clinical instability, early ultrasound dysplasia,12 and acetabular dysplasia in adolescence or adulthood.13
Our study has several limitations. It was a retrospective analysis of data collected prospectively by the Pregnancy Outcome Statistics Unit, so that other potential risk factors described in the literature, including family history, could not be analysed. The analysis of the diagnosis of dysplasia and its treatment was retrospectively performed by retrieving medical records. The severity of DDH was not graded, but all late diagnosed DDH hips were considered dislocated by the treating clinician. Our study was limited to South Australian data, and the incidence and risk factors in other states may be different.
The low published incidence rate of late DDH in SA over a number of years provides evidence that our historical screening program was successful.4 The reasons for the current increase in late diagnosed DDH in SA and elsewhere are likely to be multifactorial.
In the first instance, there is a need for greater awareness of the problem. Although there has been significant concern within the paediatric–orthopaedic community about an increase in late DDH presentations,3,5,6 awareness of this trend may not be widespread among other practitioners involved in hip screening and early child care. Web-based advocacy groups have been formed to promote awareness of hip dysplasia and to provide educational material and family support. These include the International Hip Dysplasia Institute14 and Australian patient advocacy groups.15,16
Most cases of DDH in SA are diagnosed clinically, and a high quality clinical hip examination of newborns by a competent examiner remains a powerful tool. However, an early neonatal hip examination that finds nothing abnormal does not always preclude DDH at a later follow-up.17 Early discharge from hospital is a risk factor for late DDH in SA; previous research found a higher incidence when mother and child were discharged less than 4 days after the birth.4 Our study found an association when data were analysed continuously rather than categorically, possibly because a greater number of mothers are now discharged less than 4 days after the birth. A potential reason underlying this risk factor is the reduced opportunity to examine a compliant, relaxed baby before they leave hospital.
Peaks in late DDH diagnoses occur at ages 3 and 6 months (Box 2) in association with standard timings for baby hip examinations, providing further evidence of the value of repeated clinical examination until walking age.9 Increased clinician awareness of the importance of repeated hip checks is required.
Possible reasons for the increased risk of late DDH in babies born in the country include the lack of resources to ensure the availability of appropriate screening checks and reduced clinical examination skills in practitioners who routinely examine fewer baby hips than practitioners in busy metropolitan centres. Online resources, such as the education module developed by the Royal Children’s Hospital in Melbourne,18 may be particularly helpful for rural practitioners involved in neonatal care. General practitioners and other relevant health professionals should focus on a dedicated hip examination as part of their general assessment of rural babies. High quality physical examination by a trained practitioner remains the best tool for reducing the incidence of late DDH.
There is abundant epidemiological evidence for the negative effects on hip development of wrapping, swaddling or carrying susceptible babies with hips tightly adducted and extended,10,19 as well as confirmatory animal studies.20,21 There is growing concern among the orthopaedic fraternity in North America, the UK and Australia that a resurgence in the popularity of swaddling, including the increased use of “swaddling cocoons” (which force the lower limbs into extension), places children at risk of late diagnosed DDH.3,10,19 This increased popularity has occurred following promotion of swaddling as a technique for settling babies and for reducing the risk of sudden infant death syndrome.19 Although swaddling is more common in certain cultures,10 changes in immigration trends in Australia are unlikely to account significantly for the increase in late DDH cases; in our study, only nine babies with late DDH were born to non-European mothers. Conversely, childcare practices in some cultures that encourage flexion and abduction of newborn hips, such as baby-wearing, are associated with low rates of DDH.22 Advice regarding healthy hip swaddling, as promoted by the International Hip Dysplasia Institute,14 should be provided to all new parents and practices, including advice about carrying babies with hips flexed and abducted.
The increase in late DDH cases in Australia and abroad contrasts with the experience of centres that practise universal ultrasound screening of all neonates.1,2 Evidence for the value of universal ultrasound screening includes reduced rates of surgery, hospitalisation, and late diagnoses in screened children.1,2 A variety of ultrasound methods have been successfully employed for screening, and, although variation in interpretation has been reported, this tends to occur with degrees of dysplasia of lesser clinical relevance.23 Arguments against universal ultrasound screening include variability in technique and reporting, increased follow-up, risk of overtreatment, and the acknowledgement that many abnormalities detected by ultrasound resolve spontaneously, particularly in the presence of a normal clinical examination.24 Additionally, late dysplasia can still occur in patients with a normal early screening ultrasound.25 In SA, the previously low published rates of late DDH could be considered as evidence against the need for universal ultrasound screening, but further investigation is warranted if late DDH rates remain high despite the adoption of other proposed strategies, including avoiding lower limb wrapping.
In Japan, a systematic program of public education that aimed to eliminate the use of traditional swaddling, together with education of medical practitioners and the introduction of a clinical screening program, was successful in reducing the incidence of infantile hip dysplasia from 5–6% to less than 0.4%.21 We can therefore be optimistic that the recent increase in the incidence of late diagnosed DDH can be reversed in SA and other Australian states by increasing awareness and education, and by reducing childcare practices that may be detrimental to hip development.
Box 1 – Incidence of late diagnosed cases of developmental dysplasia of the hip in babies born 1988–2009 in South Australia
Box 2 – Age of children (born 1988–2009 in South Australia) diagnosed with late developmental dysplasia of the hip
Box 3 – Univariable and multivariable analysis of risk factors associated with late developmental dysplasia (DDH) of the hip in babies born 1988–2009 in South Australia
Characteristic |
Late DDH |
Early DDH |
Unadjusted odds ratio (95% CI) |
P |
Adjusted odds ratio* (95% CI) |
P |
|||||||||
Number |
101 |
777 |
|||||||||||||
Mother’s ethnic background |
|||||||||||||||
European descent |
92 |
725 |
1.00 |
||||||||||||
Other |
9 |
52 |
1.36 (0.65–2.86) |
0.411 |
|||||||||||
Hospital category |
|||||||||||||||
Country hospital |
23 |
93 |
2.65 (1.49–4.70) |
0.001 |
2.47 (1.37–4.46) |
0.003 |
|||||||||
Home birth |
1 |
1 |
– |
– |
|||||||||||
Public metropolitan hospital |
35 |
375 |
1.00 |
1.00 |
|||||||||||
Private metropolitan hospital |
42 |
308 |
1.46 (0.91–2.35) |
0.116 |
1.41 (0.87–2.29) |
0.163 |
|||||||||
Sex |
|||||||||||||||
Female |
90 |
621 |
2.06 (1.07–3.94) |
0.03 |
1.75 (0.90–3.40) |
0.098 |
|||||||||
Male |
11 |
156 |
1.00 |
1.00 |
|||||||||||
Parity |
|||||||||||||||
First-born |
49 |
462 |
1.00 |
1.00 |
|||||||||||
Second-born |
45 |
207 |
2.05 (1.32–3.17) |
0.001 |
1.69 (1.08–2.66) |
0.023 |
|||||||||
Third-born |
7 |
75 |
0.88 (0.38–2.02) |
0.762 |
0.62 (0.25–1.53) |
0.3 |
|||||||||
Fourth-born or later |
0 |
33 |
— |
||||||||||||
Presentation |
|||||||||||||||
Cephalic |
92 |
547 |
1.00 |
1.00 |
|||||||||||
Breech |
8 |
220 |
0.22 (0.10–0.45) |
< 0.001 |
0.25 (0.12–0.54) |
< 0.001 |
|||||||||
Other |
1 |
8 |
0.74 (0.09–6.01) |
0.781 |
0.99 (0.12–8.16) |
0.989 |
|||||||||
Unknown |
0 |
2 |
— |
||||||||||||
Delivery |
|||||||||||||||
Spontaneous vaginal |
47 |
291 |
1.00 |
||||||||||||
Assisted vaginal |
16 |
95 |
1.04 (0.56–1.92) |
0.893 |
|||||||||||
Breech |
0 |
12 |
— |
||||||||||||
Elective caesarean delivery |
20 |
193 |
0.64 (0.37–1.12) |
0.116 |
|||||||||||
Emergency caesarean delivery |
18 |
186 |
0.60 (0.34–1.06) |
0.08 |
|||||||||||
Post-natal days in hospital |
|||||||||||||||
< 4 days |
36 |
225 |
1.00 |
||||||||||||
≥ 4 days |
65 |
552 |
0.74 (0.48–1.14) |
0.168 |
|||||||||||
Baby weight |
|||||||||||||||
< 2500 g |
6 |
24 |
1.92 (0.76–4.83) |
0.166 |
|||||||||||
2500–3999 g |
87 |
668 |
1.00 |
||||||||||||
≥ 4000 g |
8 |
85 |
0.72 (0.34–1.54) |
0.401 |
|||||||||||
Gestation |
|||||||||||||||
< 37 weeks |
4 |
39 |
0.77 (0.27–2.22) |
0.636 |
|||||||||||
37–41 weeks |
97 |
734 |
1.00 |
||||||||||||
≥ 42 weeks |
0 |
4 |
— |
||||||||||||
Maternal age |
|||||||||||||||
< 25 years |
12 |
98 |
1.20 (0.57–2.52) |
0.636 |
|||||||||||
25–29 years |
22 |
215 |
1.00 |
||||||||||||
30–34 years |
44 |
280 |
1.54 (8.90–2.64) |
0.121 |
|||||||||||
≥ 35 years |
23 |
184 |
1.22 (0.66–2.26) |
0.525 |
|||||||||||
* Multivariable logistic regression model including predictors with a trend to significance (P < 0.1), using backward selection. | |||||||||||||||
Received 23 September 2015, accepted 21 January 2016
- Kathrin Studer1
- Nicole Williams1,2
- Georgia Antoniou1
- Catherine Gibson3
- Heather Scott3
- Wendy K Scheil4
- Bruce K Foster1,2
- Peter J Cundy1,2
- 1 Women's and Children's Hospital, Adelaide, SA
- 2 University of Adelaide Centre for Orthopaedic and Trauma Research, Adelaide, SA
- 3 South Australian Birth Defects Register, Women's and Children's Hospital, Adelaide, SA
- 4 Pregnancy Outcomes Statistics Unit, SA Health, Adelaide, SA
The authors would like to thank the Bone Health Foundation and Big W for providing institutional research funding support.
Nicole Williams, Bruce Foster and Peter Cundy are medical advisers to the International Hip Dysplasia Institute. Bruce Foster is the Healthy Hips Australia patron.
- 1. Tschauner C, Furntrath F, Saba Y, et al. Developmental dysplasia of the hip: impact of sonographic newborn hip screening on the outcome of early treated decentered hip joints-a single center retrospective comparative cohort study based on Graf’s method of hip ultrasonography. J Child Orthop 2011; 5: 415-424.
- 2. Thallinger C, Pospischill R, Ganger R, et al. Long-term results of a nationwide general ultrasound screening system for developmental disorders of the hip: the Austrian hip screening program. J Child Orthop 2014; 8: 3-10.
- 3. Williams N, Foster BK, Cundy PJ. Is swaddling damaging our babies’ hips? Med J Aust 2012; 197: 272. <MJA full text>
- 4. Azzopardi T, Van Essen P, Cundy PJ, et al. Late diagnosis of developmental dysplasia of the hip: an analysis of risk factors. J Pediatr Orthop B 2011; 20: 1-7.
- 5. Birke O, Wallgren L, Bridge C, et al. Late open reduction and pelvic ± femoral osteotomy for increasing numbers of missed walking age DDH hip dislocation vs early treatment with Pavlik harness — evaluation at 5 years follow-up [unpublished presentation]. Australian Paediatric Orthopaedic Society annual scientific meeting, Noosa (Australia), 22 Sept 2012.
- 6. Lisle R, Boekelaar M, Stannage K, Whitewood C. Delayed diagnosis of developmental dislocation of the hip: the Western Australian experience. ANZ J Surg 2012; 82: 612-615.
- 7. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip; American Academy of Pediatrics. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics 2000; 105: 896-905.
- 8. Wicart P, Bocquet A, Gelbert N, et al. Congenital dislocation of the hip: optimal screening strategies in 2014. Orthop Traumatol Surg Res 2014; 100(6 Suppl): S339-S347.
- 9. Price KR, Dove R, Hunter JB. Current screening recommendations for developmental dysplasia of the hip may lead to an increase in open reduction. Bone Joint J 2013; 95-B: 846-850.
- 10. Clarke NM. Swaddling and hip dysplasia: an orthopaedic perspective. Arch Dis Child 2014; 99: 5-6.
- 11. Price CT. Swaddling and hip dysplasia: new observations. Commentary on an article by Enbo Wang, MD, PhD, et al.: “Does swaddling influence developmental dysplasia of the hip? An experimental study of the traditional straight-leg swaddling model in neonatal rats”. J Bone Joint Surg Am 2012; 94: e92.
- 12. Shorter D, Hong T, Osborn DA. Cochrane review: screening programmes for developmental dysplasia of the hip in newborn infants. Evid Based Child Health 2013; 8: 11-54.
- 13. Lee CB, Mata-Fink A, Millis MB, Kim YJ. Demographic differences in adolescent-diagnosed and adult-diagnosed acetabular dysplasia compared with infantile developmental dysplasia of the hip. J Pediatr Orthop 2013; 33: 107-111.
- 14. International Hip Dysplasia Institute. Hip-healthy swaddling [video]. http://hipdysplasia.org/developmental-dysplasia-of-the-hip/hip-healthy-swaddling/ (accessed Sept 2015).
- 15. Hip Hip Hooray: raising awareness for hip dysplasia [website]. http://www.hiphiphoorayddh.org/ (accessed Sept 2015).
- 16. Healthy Hips Australia [website]. http://www.healthyhipsaustralia.org.au/ (accessed Sept 2015).
- 17. Raimann A, Baar A, Raimann R, Morcuende JA. Late developmental dislocation of the hip after initial normal evaluation: a report of five cases. J Pediatr Orthop 2007; 27: 32-36.
- 18. Donnan L, The Royal Children’s Hospital Melbourne. Developmental dysplasia of the hip [website, video]. Melbourne: Department of Education and Early Childhood Development (Victoria), 2011. http://www.ddheducation.com/ (accessed Sept 2015).
- 19. Mahan ST, Kasser JR. Does swaddling influence developmental dysplasia of the hip? Pediatrics 2008; 121: 177-178.
- 20. Wang E, Liu T, Li J, et al. Does swaddling influence developmental dysplasia of the hip? An experimental study of the traditional straight-leg swaddling model in neonatal rats. J Bone Joint Surg Am 2012; 94: 1071-1077.
- 21. Yamamuro T, Ishida K. Recent advances in the prevention, early diagnosis, and treatment of congenital dislocation of the hip in Japan. Clin Orthop Relat Res 1984; (184): 34-40.
- 22. Graham SM, Manara J, Chokotho L, Harrison WJ. Back-carrying infants to prevent developmental hip dysplasia and its sequelae: is a new public health initiative needed? J Pediatr Orthop 2015; 35: 57-61.
- 23. Roposch A, Graf R, Wright JG. Determining the reliability of the Graf classification for hip dysplasia. Clin Orthop Relat Res 2006; 447: 119-124.
- 24. Tegnander A, Holen KJ, Terjesen T. The natural history of hip abnormalities detected by ultrasound in clinically normal newborns: a 6–8 year radiographic follow-up study of 93 children. Acta Orthop Scand 1999; 70: 335-337.
- 25. Imrie M, Scott V, Stearns P, et al. Is ultrasound screening for DDH in babies born breech sufficient? J Child Orthop 2010; 4: 3-8.





Susan LyndsayCharlton
In the Limestone Coast region of South Australia research has been carried out over the last four years into methods of assessment of infant hips with a view to providing better education about assessment and management of neonatal instability of the hip. One preliminary pilot study (Charlton 2012) offered early anterior dynamic ultrasound (ADUS) for hip screening to all infants born at a regional hospital. This study demonstrated ADUS was logistically feasible with high parent acceptance in a regional hospital
Following on from this study we have provided early ADUS to all babies with such risk factors as family history, breech delivery or abnormal clinical assessment. We have a cohort of 116 babies in this group since September 2013 and of that number, 22 warranted follow up ultrasound at 6 weeks. Four infants went on to splinting for insufficient femoral head cover on Graf ultrasound.
A further study is being conducted exploring whether ADUS has a place in educating and motivating parents to engage in preventative strategies such as loose swaddling and prone positioning of their baby.
A break down by postcode or region on the statistics in the published study would be of interest in possible variation in incidence of late diagnosis between regions in the light of these studies.
Competing Interests: No relevant disclosures
Mrs Susan LyndsayCharlton
Flinders University Rural Clinical School
Nicole Williams
This study included data on South Australian births up to 2009, to allow a full 5 year follow-up to capture a late diagnosis. Any initiatives since then would not be reflected in this data.
To help with your ongoing efforts, we can provide the following information: of the 23 babies born in South Australian rural and regional hospitals with late DDH, 6 were from the Limestone Coast. The percentage of all cases of DDH born in Limestone Coast Hospitals from 2003-2009 with a late diagnosis was 22%, which is slightly above the figure for rural and regional births as a whole as presented in Box 3 in the article.
This study provides further evidence that selective ultrasound screening based on risk factors fails to prevent late diagnosed cases as many children will not have traditional risk factors. We continue to promote careful clinical examination in all babies and ask practitioners to be mindful that if performing ultrasound screening based on risk factors, this will fail to identify the cephalic presentation, second-born babies we found to be at risk of late diagnosis in this study.
Competing Interests: Study corresponding author
Dr Nicole Williams
Women's and Children's Hospital, North Adelaide