Osteoporosis is characterised by low bone mineral density (BMD) and micro‐architectural deterioration of bone tissue, leading to decreased bone strength and increased fragility and fracture risk. Osteoporotic (fragility) fractures usually follow falls from a standing height, or less, in individuals with decreased bone strength, and involve low or minimal trauma. Bone mineral density can be reliably measured by scanning the skeleton using dual‐energy x‐ray absorptiometry (DXA). Deterioration of skeletal tissue proceeds with no symptoms until a symptomatic fracture occurs. The condition is therefore under‐recognised and affected individuals are undertreated.1,2
Fracture‐related morbidity can arise from pain, reduced mobility, loss of function, and consequent reduced quality of life.3 Many patients are unable to live independently following a hip fracture. Long term morbidity is associated with symptomatic osteoporotic fractures at almost all anatomic sites.3 Mortality in the first year after a major minimal trauma fracture in people aged over 60 years is up to threefold higher than in age‐matched non‐fracture populations for people with hip fracture and up to twofold higher for other major fracture types4,5 — major fractures include those of the pelvis, hip, distal forearm, humerus, and vertebrae. Prompt diagnosis and optimal treatment of osteoporosis prevents further fractures and reduces mortality.6,7,8
The Australian Government Department of Health and Aged Care contracted Healthy Bones Australia (formerly Osteoporosis Australia), which is a national not‐for‐profit organisation and a leading national bone health consumer body, to update the previous Royal Australian College of General Practitioners (RACGP) and Osteoporosis Australia (now, Healthy Bones Australia) 2017 guideline for osteoporosis management.9 The accumulation of high quality evidence supporting improvements in clinical practice over the past five years, the need for expert consensus and opinion, and new developments in pharmacological management of osteoporosis, especially the role of osteoanabolic therapies such as romosozumab and teriparatide, prompted this update.
The updated guideline10 was designed to provide clear, evidence‐based recommendations to assist Australian general practitioners in managing patients over 50 years of age with poor bone health (osteopenia and osteoporosis). Its purpose was to support, not replace, clinical decision making in the individual patient, and to assist busy general practitioners in achieving better patient outcomes by:
- preventing the first fracture;
- diagnosing osteoporosis early to allow prompt bone health management;
- identifying undiagnosed patients following a first fracture to prevent subsequent fractures; and
- managing secondary causes of poor bone health.
Methods
Recommendations in the previous (second) edition9 were based on critical analysis of published, peer‐reviewed evidence from 2006 to 2016, following a systematic review of available evidence. Every section in the updated guideline10 was reviewed and updated by a Subject Matter Adviser (Supporting Information, table 1) with subspecialty expertise in that topic using new peer‐reviewed evidence published from 2017. Focused literature searches were undertaken in subject areas that the Guideline Review Committee (PW, WC, DE, CG, MR, JT, JW) felt needed particular attention. These included fracture risk assessment tools, the frequency of DXA monitoring, patients at “imminent” or “very high” fracture risk, and pharmacological therapies. For these areas, the Guideline Review Committee provided specific keywords to the RACGP team, who searched the following databases: PubMed, Medline, National Institute for Heath and Care Excellence (NICE), Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (CENTRAL), Scottish Intercollegiate Guidelines Network (SIGN), Trip Database, and Google. Filters were applied in Ovid Medline to identify randomised controlled trials, systematic reviews and meta‐analyses. Other filters applied included men and women older than 45 years of age and studies reporting outcomes of fracture and/or BMD. As far as possible, evidence to support recommendations covering pharmacological and other therapeutic interventions was restricted to studies with fracture as a primary outcome. However, for some interventions, evidence meeting this criterion was sparse, or of variable quality, and high quality studies with BMD as a primary outcome were used if, in the opinion of the Guideline Review Committee, the data could be used to support recommendations.
Each of the 45 recommendations was given a final grading from A to D according to the National Health and Medical Research Council grades of recommendation.11 The grading represents the overall strength of evidence and reflects the confidence with which clinicians can apply a recommendation in a clinical situation. However, they should be used in conjunction with clinical judgement and individual patient context and preferences. The recommendations do not cover complex medical conditions with comorbidities, nor are they a substitute for individualised specialist advice and/or consultation, which may be required for optimal patient care.
Where insufficient evidence was available, or where the quality of evidence did not meet minimum requirements, recommendations were developed through the Guideline Review Committee's unanimous consensus, cognisant of the complexities and time constraints of a busy general practitioner.
Conflicts of interest
All members of the Guideline Review Committee were asked to declare any potential conflicts of interest. This was updated at each meeting and is available as a supplementary document.10 The management of the conflicts of interest was undertaken as per Guideline International Network (GIN)12 principles and are explained in the supplementary document.10
Consultation and endorsement by the RACGP
Due to resource and time restrictions, consultation was focused on Healthy Bones Australia, stakeholders and review by the intended guideline users, namely general practitioners. The Guideline Review Committee was particularly aware of the importance of clear and pragmatic advice for busy general practitioners in everyday clinical practice. This guide was reviewed by general practice subject matter experts (DE, MR, JT) on the Guideline Review Committee and the RACGP's Expert Committee – Quality Care and endorsed by the RACGP Board.
Recommendations
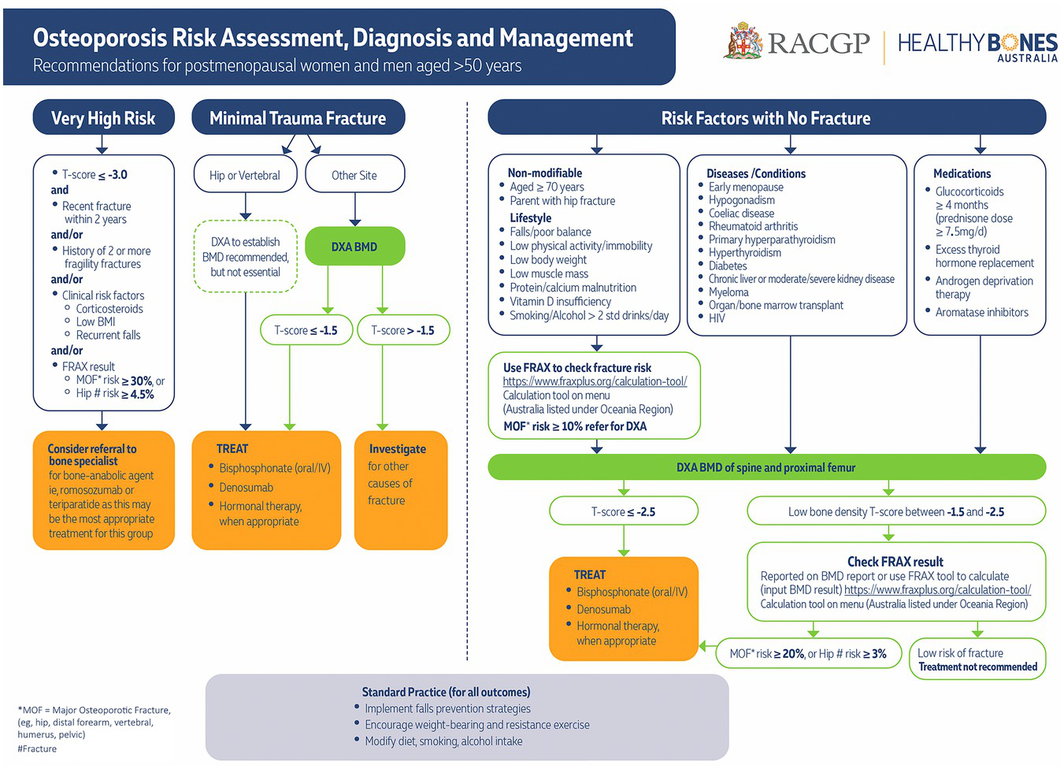
The guideline recommendations are outlined in Box 1. The full guideline is freely available at https://www.racgp.org.au/clinical‐resources/clinical‐guidelines/key‐racgp‐guidelines/view‐all‐racgp‐guidelines/osteoporosis/executive‐summary. A high resolution summary flowchart is available at https://www.racgp.org.au/getattachment/f31c6529‐96f0‐4840‐8f41‐c98bd5e4fad7/attachment.aspx?disposition=inline (Box 2). Significant updates since the 2017 guideline are discussed next.
Clinical implications of updated recommendations
Absolute risk calculation
Feedback received from general practitioners regarding the previous guideline was that they needed clear advice regarding which fracture absolute risk calculator to use in routine clinical practice. The Guideline Review Committee recognised the limitations of the FRAX tool (https://FRAXplus.org/calculation‐tool),13,14 in particular, the use of binary (yes/no) responses for some inputs, the exclusion of falls as an input, and the lack of an open source calculation algorithm.15 However, many international guidelines suggest its use16,17 because of validation in multiple populations, inclusion in many DXA reports, the regular algorithm refinement following user feedback,18 and the consideration of death as a competing hazard, meaning that fracture risk is reduced in people with low life expectancy (eg, older, frailer people). For these reasons FRAX was recommended, although clinical judgement remains essential for interpretation and communication of the ten‐year fracture risk output to patients. However, the simplicity of the Garvan Fracture Risk Calculator (only five input factors), which includes falls as one of the inputs, makes it very convenient, especially for patients who experience a fall.19
Case finding
Since the previous guideline edition, there have been three large population‐based randomised controlled trials of screening in women for prevention of osteoporotic fractures: Screening in the Community to Reduce Fractures in Older Women (SCOOP) in the United Kingdom,20 Risk‐stratified Osteoporosis Strategy Evaluation (ROSE) in Denmark,21 and the SALT Osteoporosis Study (SOS) in The Netherlands.22 Although none showed a statistically significant reduction in the primary outcome of all fractures, there was a trend to a reduction. The planned secondary endpoint of a reduction in hip fractures showed a significant result in one trial and consistent non‐statistically significant improvements in the other two. This resulted in a significant result for hip fracture reduction in a meta‐analysis (total number of patients included, > 42 000).23 Although promising, optimal thresholds of absolute fracture risk and implementation strategies are inadequately defined for Australia and there are no data on screening in men. Therefore, the Guideline Review Committee concluded that there is insufficient evidence to support a population‐based screening program in Australia.
A rational approach to assessment and BMD testing
Risk factors can be considered to better understand an individual's risk through the use of a fracture absolute risk calculator (Box 2). The screening trials used a two‐step process of initial FRAX risk assessment guiding the need for BMD measurement by DXA followed by repeat FRAX risk assessment, incorporating the BMD reading obtained at DXA to guide treatment recommendations.20,21,22 Use of a risk estimation tool, such as FRAX, also removes the need to set different minimum ages for initial risk enquiry for men and women, as sex and age are part of the risk estimation algorithm.
The absolute risk at which to recommend DXA and the threshold for commencement of pharmacotherapy is important, yet not consistently defined. The level of risk perceived as “high” will vary between individuals and will differ depending on regional regulatory body funding. Clinical trial results enable an estimate of absolute risk at which treatment is effective. In the Fracture Intervention Trial (FIT), where oral alendronate was effective at reducing fractures, almost all patients had a baseline ten‐year fracture risk greater than 10%.24 In the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every Six Months (FREEDOM) trial of denosumab, which is a monoclonal antibody that inhibits receptor activator of nuclear factor‐κβ ligand [RANKL]), the median baseline ten‐year fracture risk was 15%.25 A trial of zoledronate–zoledronic acid in women with osteoporosis over 65 years old was effective, with a median baseline absolute risk of 12% for fracture at ten years.26
The thresholds used in the screening trials can also inform this choice. The ROSE study21 used a ten‐year fracture risk (FRAX) threshold of 15% to recommend DXA testing. The SCOOP20 trial used a range of age‐specific thresholds (3.4% at 50 years, rising to an 11.1% ten‐year risk of major osteoporotic fracture at 70 years), which might make implementation in the Australian primary care setting difficult without clinical decision support software for risk thresholds by age.
Given that case finding would be used for a population selected for their interest to engage in fracture prevention interventions, the impact can be expected to be better than demonstrated in population screening trials. A slightly lower threshold for recommending BMD has been adopted, as was done in the SIGN guidelines 2021, where a ten‐year risk of major osteoporotic fracture of more than 10% triggers a recommendation for BMD measurement, which is relatively pragmatic and inclusive.27 Patient preferences and value placed on a risk estimate should also guide further management.
A helpful guide to DXA testing, including appropriate Medicare Benefits Schedule item numbers, is provided in the guideline (appendix C)10 and is shown in the Supporting Information, figure 1.
Imminent and very high fracture risk
The concept of imminent and very high fracture risk is evolving. The increased risk of refracture within the first 24 months following incident fracture means this is a crucial period in which to perform bone health assessment. Identifying patients with “very high” fracture risk is important with the increasing availability of osteoanabolic therapy, as these patients are a logical group in which to consider the use of these agents as initial therapy, subject to regulatory and funding limitations. The following features provide a broad guide as to which patients might be at “very high” fracture risk: (i) recent fracture and a ten‐year FRAX major osteoporotic fracture risk of 30% or over;16 (ii) a recent fracture (within 12 months); (iii) a T‐score below ‐3.0; (iv) multiple fractures while on therapy; (v) the use of drugs causing skeletal harm; and (vi) a ten‐year FRAX major osteoporotic fracture risk of 30% or above, or hip fracture risk of more than 4.5%.17 As outlined in the summary flowchart (Box 2), and available at https://www.racgp.org.au/getattachment/f31c6529‐96f0‐4840‐8f41‐c98bd5e4fad7/attachment.aspx?disposition=inline, such patients should be considered for referral to a bone specialist for consideration of early osteoanabolic therapy (romosozumab or teriparatide) followed by antiresorptive therapy.
Calcium, vitamin D and protein supplementation
The use of calcium, vitamin D and protein supplementation is a complex area, with a vast amount of published literature discussed in the updated guideline.10 The absolute benefit of calcium and vitamin D supplementation for short term (less than five years) fracture prevention for non‐institutionalised individuals is relatively low and much less than with pharmacological treatments, such as bisphosphonates or denosumab.28,29 The United States Preventive Services Task Force has recommended against routine calcium and vitamin D supplementation in non‐institutionalised older people.30 However, a comprehensive umbrella review concluded there was reasonable benefit for those who may be deficient, especially in institutionalised individuals or frail older people.31
The target calcium intake from dietary sources and supplements should be 1000 mg per day for adults, rising to 1300 mg per day for women aged over 50 years and men aged over 70 years.28,32,33 Vitamin D can be obtained from sunlight exposure or, if sun exposure is limited, supplements should ensure a serum 25‐hydroxyvitamin D (25(OH)D) concentration of more than 50 nmol/L.28 If oral vitamin D supplements are required,28 a dose of 800–1000 IU per day is usually sufficient.
Calcium supplements modestly increase the risk of renal calculi and can cause abdominal bloating and constipation.34 Although an increased risk of myocardial infarction with calcium supplements has been reported,35 not all studies support this conclusion.36,37 However, obtaining an adequate calcium intake by dietary means is preferable.
Findings from the large Vitamin D and Omega‐3 Trial (VITAL) study were recently published and cast doubt on the role of vitamin D3 supplementation in fracture risk reduction.38 However, this study was done in generally healthy mid‐life and older adults who were not selected for vitamin D deficiency, low bone mass, or osteoporosis.
Based on a large body of evidence over many years, calcium and vitamin D supplements are more likely to be effective in reducing fracture risk when given in combination to individuals who are deficient (serum 25(OH)D < 50 nmol/L). It is important to note that most pharmacological intervention studies were done in calcium‐ and vitamin D‐replete individuals. In healthy non‐institutionalised individuals, the relative reduction in fracture risk with calcium and/or vitamin D supplementation alone is small and, thus, these should not be considered for routine use in healthy people or as first line treatment for people with osteoporosis.
An authoritative position statement, Balancing the harms and benefits of sun exposure,39 by the Australian Skin and Skin Cancer Research Centre and endorsed by a wide range of stakeholders, was released as this guideline was being updated. This provides practical advice about the duration of sunlight exposure required for adequate skin production of vitamin D (vitamin D‐effective dose of sunlight) in people with diverse skin tones residing in various geographic locations around Australia. As the risks and benefits of sun exposure are mainly determined by skin type and risk of skin cancer, the recommendations are stratified as follows:
- Individuals at high risk of skin cancer (eg, those with very pale skin). In this group, time outdoors with an ultraviolet (UV) index (a measure of UV radiation ranging from 0 [low] to 11 [extremely high]) ≥ 3 should be avoided, and if outdoors at those times, full sun protection measures should be implemented — “Slip on covering clothing. Slop on sunscreen with a sun protection factor [SPF] ≥ 30. Slap on a hat. Seek shade. Slide on sunglasses”.
- Individuals at low risk of skin cancer (eg, those with dark skin). These people should be advised to spend sufficient time outdoors with enough skin exposed when the UV index is ≥ 3.
- Individuals at intermediate risk of skin cancer (eg, those with olive or pale brown skin and no other risk factors). These people should be advised to spend enough time outdoors with sufficient skin exposed for a vitamin D‐effective dose of sunlight. Full sun protection measures (see above) should be used if spending more time than that required to obtain a vitamin D‐effective dose.39
Readers requiring exposure times for a vitamin D‐effective dose of sunlight should refer to the detailed seasonal charts in the position statement for their specific geographic area of practice.39
The importance of protein supplementation was highlighted by an influential Melbourne study that assessed the effectiveness of a nutritional intervention in institutionalised older adults by improving calcium and protein intake (< 1 g/kg body weight protein per day) using dairy foods. This study showed an 11% reduction in falls risk, a 48% reduction in hip fractures, and a 30% reduction in all fractures in the intervention group.40
In summary, supplementation with calcium (target intake > 1300 mg per day), vitamin D (target serum 25(OH)D > 50 nmol/L) and protein (1–1.2 g/kg body weight per day) should be targeted to people who need it most, namely frail, institutionalised individuals, especially those receiving bone‐protective therapy.
Osteoanabolic therapy
The current Pharmaceutical Benefit Scheme (PBS)‐subsidised indications for bone protective therapy are outlined in the Supporting Information, table 2.41 Therapeutic options for poor bone health have changed since the previous guideline, with removal of strontium ranelate due to associated excess cardiovascular mortality,42 and addition of the sclerostin inhibitor, romosozumab, which increases bone formation and reduces bone resorption. This novel dual mechanism of action leads to a marked increase in BMD, greater than that seen with oral alendronate (bisphosphonate) or teriparatide (recombinant human parathyroid hormone (1‐34) [rhPTH (1‐34)]).43,44 Concern about a small increase in cardiovascular adverse events compared with alendronate in one randomised controlled trial45 with several local notifications of adverse events prompted the Therapeutic Goods Administration to issue an alert that romosozumab should be avoided in individuals with previous myocardial infarction or stroke.46 As always, its use requires discussion with the individual patient regarding risk and benefit, especially as the same ageing population at risk of poor bone health is also at increased risk of adverse cardiovascular events.
The other osteoanabolic agent available via the PBS subsidy is rhPTH (1‐34), teriparatide (Supporting Information, table 2). Withdrawal of the originator compound, Forteo (Eli Lilly) has left the biosimilars Terrosa (Gedeon Richter) and Teriparatide Lupin (Generic Health) as the only teriparatide formulations in Australia. Prescription (total duration of therapy, 18 months) can be continued by a general practitioner following initiation by a bone specialist.
Wider access to osteoanabolic therapies (romosozumab and rhPTH (1‐34)) has prompted identification of patients at “very high” fracture risk (see above and Box 2), as these should be considered for early osteoanabolic therapy followed by an antiresorptive agent, subject to regulatory and funding restrictions.
As of 1 November 2024, romosozumab received a first line PBS listing for patients at very high fracture risk — the detailed statement of the PBS indication is presented in the Supporting Information, table 2.41 This will allow initiation of potent bone anabolic therapy in treatment‐naïve patients to be sequentially followed by antiresorptive therapy (eg, a bisphosphonate or denosumab) to achieve and maintain the greatest possible gain in BMD.45
Transition of bone protective agents
Although the advent of denosumab has been a major advance in the treatment of osteoporosis, its discontinuation, or even delaying the injection by more than four months can be associated with rebound bone resorption and vertebral fractures.47,48 Even though definitive measures to prevent this remain unclear, denosumab should either be continued long term or its cessation followed by an antiresorptive medication; for example, 12 months of an oral bisphosphonate or one or more infusions of zoledronate–zoledronic acid (a potent intravenous bisphosphonate).49,50 Most general practices have a robust recall system to ensure denosumab administration occurs at the specified six‐monthly intervals to minimise the risk of rebound vertebral fractures.
Implementation
The complete guideline can be accessed at no cost from the following professional society websites:
- RACGP (https://www.racgp.org.au/clinical‐resources/clinical‐guidelines/key‐racgp‐guidelines/view‐all‐racgp‐guidelines/osteoporosis/executive‐summary);
- Australian Rheumatology Association (https://rheumatology.org.au/For‐Healthcare‐Professionals/Clinical‐Resources/Other); and
- Australian and New Zealand Bone and Mineral Society (https://www.anzbms.org.au/policies.asp).
It can also be accessed from the Healthy Bones Australia website (https://healthybonesaustralia.org.au/health‐care‐professionals/gp‐resources/).
Conclusion
Poor bone health (osteopenia and osteoporosis) is highly treatable with appropriate widely available lifestyle and dietary measures and pharmacological agents. As general practice is the only extensive workforce capable of long term care of patients with osteoporosis, supporting general practitioners to manage osteoporosis is critical. The updated guideline is designed to be an evidence‐based pragmatic tool to assist general practitioners in the day‐to‐day care of such patients in partnership with bone specialists.
Box 1 – Recommendations10
|
Section |
No. |
Recommendation |
Grade* |
||||||||||||
|
|
|||||||||||||||
|
1. Risk factors, fracture risk assessment and case finding |
|||||||||||||||
|
1.1 Identifying patients to investigate for osteoporosis |
1 |
|
A |
||||||||||||
|
2† |
|
A |
|||||||||||||
|
3 |
|
B |
|||||||||||||
|
1.2 Measurement of bone mineral density |
4† |
|
A |
||||||||||||
|
1.3 Assessment of absolute fracture risk |
5† |
|
B |
||||||||||||
|
6† |
|
C |
|||||||||||||
|
1.4 Case finding |
7 |
|
A |
||||||||||||
|
8† |
|
D |
|||||||||||||
|
9† |
|
C |
|||||||||||||
|
10 |
|
B |
|||||||||||||
|
2. General bone health maintenance and fracture prevention |
|||||||||||||||
|
2.1 Calcium, protein and vitamin D |
11† |
For generally healthy older people:
|
C |
||||||||||||
|
12† |
For frail and institutionalised older people:
|
B |
|||||||||||||
|
13† |
For people taking osteoporosis treatments:
|
C |
|||||||||||||
|
14† |
|
B |
|||||||||||||
|
2.2 Reducing falls |
15 |
|
A |
||||||||||||
|
16 |
|
A |
|||||||||||||
|
2.3 Exercise |
17 |
Exercises recommended to reduce fracture risk:
|
B |
||||||||||||
|
|
18 |
|
C |
||||||||||||
|
|
19 |
|
B |
||||||||||||
|
|
20 |
|
D |
||||||||||||
|
3. Pharmacological approaches to prevention and treatment |
|||||||||||||||
|
3.1 Bisphosphonates |
21† |
|
B |
||||||||||||
|
22† |
|
A (women), C (men) |
|||||||||||||
|
23† |
|
D |
|||||||||||||
|
3.2 Denosumab |
24† |
|
A |
||||||||||||
|
25† |
|
B |
|||||||||||||
|
26† |
|
C |
|||||||||||||
|
3.3 Romosozumab |
27† |
|
A |
||||||||||||
|
28† |
|
C |
|||||||||||||
|
3.4 Menopausal hormone therapy |
29† |
|
A |
||||||||||||
|
30† |
|
A |
|||||||||||||
|
3.5 Recombinant human parathyroid hormone |
31 |
|
A |
||||||||||||
|
32 |
|
C |
|||||||||||||
|
4. Ongoing monitoring |
|||||||||||||||
|
4.1 Ongoing monitoring |
33 |
|
C |
||||||||||||
|
34 |
|
C |
|||||||||||||
|
35 |
|
D |
|||||||||||||
|
5. Special issues |
|||||||||||||||
|
5.1 Management of osteoporosis in frail and older people (> 75 years of age) |
36 |
|
C |
||||||||||||
|
5.2 Bone loss associated with aromatase inhibitor therapy for breast cancer and androgen deprivation therapy for prostate cancer |
37 |
|
A |
||||||||||||
|
38 |
|
A |
|||||||||||||
|
39 |
|
D |
|||||||||||||
|
40 |
|
C |
|||||||||||||
|
41 |
|
A |
|||||||||||||
|
42 |
|
A |
|||||||||||||
|
43 |
|
C |
|||||||||||||
|
44 |
|
C |
|||||||||||||
|
5.3 Medication‐related osteonecrosis of the jaw (MRONJ) |
45† |
|
C |
||||||||||||
|
|
|||||||||||||||
|
* National Health and Medical Research Council grades of recommendations: A = body of evidence can be trusted to guide practice; B = body of evidence can be trusted to guide practice in most situations; C = body of evidence provides some support for recommendations, but care should be taken in its application; D = body of evidence is weak and recommendations must be applied with caution. † Recommendations underwent a focused and detailed search of the published literature during which multiple databases were interrogated to identify publications subsequent to the previous edition (ie, since 2016). These were then reviewed by a Subject Matter Adviser (Supporting Information, table 1) with subspecialty topic expertise and the relevant chapters updated. The final draft of the chapters underpinning relevant recommendations was then reviewed by the Guideline Review Committee (PW, WC, DE, CG, MR, JT, JW) and discussed at several face‐to‐face and online meetings. All other recommendations have been updated by at least one Subject Matter Adviser with subspecialty expertise in the area and reviewed by the Guideline Review Committee at two face‐to‐face meetings. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Peter Wong1,2
- Weiwen Chen3,4
- Dan Ewald5,6
- Christian Girgis1,2
- Morton Rawlin2,7
- John Tsingos8
- Justine Waters9
- 1 Westmead Hospital, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
- 3 Garvan Institute, Sydney, NSW
- 4 St Vincent's Hospital, Sydney, NSW
- 5 University Centre for Rural Health, University of Sydney, Lismore, NSW
- 6 Lennox Head Medical Centre, Lennox Head, NSW
- 7 Macedon Medical Centre, Melbourne, VIC
- 8 Souths Juniors Medical Centre, Sydney, NSW
- 9 University of Technology Sydney, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
The Guideline Review Committee thanks the following people for their contributions to the guideline revision: Greg Lyubomirsky, Melita Daru and Rosa Camilleri (from Healthy Bones Australia), and Su San Mok and Stephan Groombridge (from the Royal Australian College of General Practitioners). The revised guideline was funded by the Australian Government Department of Health and Aged Care. The funding source had no role in the study design, data collection, analysis or interpretation, reporting or publication.
Peter Wong is a member of the Clinical Advisory Group, NSW Health, and Osteoporosis Refracture Working Group (no remuneration attached). He is also the site investigator for a phase 4 post‐marketing Amgen clinical trial (remuneration only to the institution). Weiwen Chen was a speaker for the educational seminar for Arrotex as part of GP Health Update Day (31 Aug 2024) and was a member of the Sandoz Advisory Board on one occasion (11 May 2024). Christian Girgis was a member of the Advisory Board for Sandoz on one occasion (11 May 2024) and was a speaker for Gedeon Richter (12 Sept 2023). All other members of the Guideline Review Committee declared no relevant competing interests.
- 1. Eisman J, Clapham S, Kehoe L; Australian BoneCare Study. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res 2009; 19: 1969‐1975.
- 2. Naik‐Panvelkar P, Norman S, Elgebaly Z, et al. Osteoporosis management in Australian general practice: an analysis of current osteoporosis treatment patterns and gaps in practice. BMC Fam Pract 2020; 21: 32.
- 3. Abimanyi‐Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int 2015; 26: 1781‐1790.
- 4. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low‐trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009; 301: 513‐521.
- 5. Bliuc D, Alarkawi D, Nguyen TV, et al. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 2014; 30: 637‐646.
- 6. Black DM, Delmas PD, Eastell R, et al. Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809‐1822.
- 7. Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta‐analysis. J Clin Endocrinol Metab 2010; 95: 1174‐1181.
- 8. Saito T, Sterbenz JM, Malay S, et al. Effectiveness of anti‐osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta‐analysis. Osteoporos Int 2017; 28: 3289‐3300.
- 9. Royal Australian College of General Practitioners and Osteoporosis Australia. Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age, 2nd ed. Melbourne: RACGP, 2017. https://healthybonesaustralia.org.au/wp‐content/uploads/2022/12/oa‐racgp‐osteoporosis‐clinical‐guidelines‐2nd‐ed.pdf (viewed Mar 2025).
- 10. Royal Australian College of General Practitioners and Healthy Bones Australia. Osteoporosis management and fracture prevention in postmenopausal women and men over 50 years of age, 3rd ed. Melbourne: RACGP, 2024. https://www.racgp.org.au/getattachment/487c5b92‐7bd0‐4b1c‐86f5‐2d86c649f92f/Osteoporosis‐management‐and‐fracture‐prevention‐in‐post‐menopausal‐women‐and‐men‐50‐years‐of‐age.aspx (viewed Mar 2025).
- 11. National Health and Medical Research Council. Guidelines for guidelines handbook. Canberra: NHMRC. http://www.nhmrc.gov.au/guidelinesforguidelines (viewed Dec 2023).
- 12. Schünemann HJ, Al‐Ansary LA, Forland F, et al. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med 2015; 163: 548‐553.
- 13. Nguyen TV. Personalised assessment of fracture risk: which tool to use? Aust J Gen Pract 2022; 51: 189‐190.
- 14. McCloskey EV, Harvey NC, Johansson H, et al. Fracture risk assessment by the FRAX model. Climacteric 2022; 25: 22‐28.
- 15. Allbritton‐King JD, Elrod JK, Rosenberg PS, Bhattacharyya T. Reverse engineering the FRAX algorithm: clinical insights and systematic analysis of fracture risk. Bone 2022; 159: 116376.
- 16. Gregson CL, Armstrong DJ, Bowden J, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2022; 17: 58.
- 17. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis — 2020, update. Endocr Pract 2020; 26: 564‐570.
- 18. Vandenput L, Johansson H, McCloskey EV, et al. Update of the fracture risk prediction tool FRAX: a systematic review of potential cohorts and analysis plan. Osteoporos Int 2022; 33: 2103‐2136.
- 19. Baim S, Blank R. Approaches to fracture risk assessment and prevention. Curr Osteoporos Rep 2021; 19: 158‐165.
- 20. Shepstone L, Lenaghan E, Cooper C, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet 2018; 391: 741‐747.
- 21. Rubin KH, Rothmann MJ, Holmberg T, et al. Effectiveness of a two‐step population‐based osteoporosis screening program using FRAX: the randomized Risk‐stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int 2018; 29: 567‐578.
- 22. Merlijn T, Swart KM, van Schoor NM, et al. The effect of a screening and treatment program for the prevention of fractures in older women: a randomized pragmatic trial. J Bone Miner Res 2019; 34: 1993‐2000.
- 23. Merlijn T, Swart KMA, van der Horst HE, et al. Fracture prevention by screening for high fracture risk: a systematic review and meta‐analysis. Osteoporos Int 2020; 31: 251‐257.
- 24. Donaldson MG, Palermo L, Ensrud KE, et al. Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the Fracture Intervention Trial. J Bone Miner Res 2012; 27: 1804‐1810.
- 25. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756‐765.
- 26. Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 2018; 379: 2407‐2416.
- 27. Scottish Intercollegiate Guidelines Network. Management of osteoporosis and the prevention of fragility fractures. SIGN, 2021. https://www.sign.ac.uk/our‐guidelines/management‐of‐osteoporosis‐and‐the‐prevention‐of‐fragility‐fractures/ (viewed Sept 2024).
- 28. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press, 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/ (viewed Oct 2023).
- 29. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta‐analysis. JAMA Netw Open 2019; 2: e1917789.
- 30. Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158: 691‐696.
- 31. Chakhtoura M, Bacha DS, Gharios C, et al. Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta‐analyses of controlled trials. J Clin Endocrinol Metab 2022; 107: 882‐898.
- 32. National Health and Medical Research Council. Australian dietary guidelines. Canberra: NHMRC, 2013 https://www.eatforhealth.gov.au/guidelines/guidelines (viewed Nov 2023).
- 33. National Institutes of Health, Office of Dietary Supplements. Calcium: fact sheet for health professionals. Bethesda (MD): NIH, 2024. https://ods.od.nih.gov/factsheets/Calcium‐HealthProfessional/ (viewed Sept 2023).
- 34. Mazess RB, Bischoff Ferrari HA, Dawson Hughes B. Vitamin D: bolus is bogus — a narrative review. JBMR Plus 2021; 5: e10567.
- 35. Bolland MJ, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ 2011; 342: d2040.
- 36. Lewis JR, Calver J, Zhu K, et al. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5‐year RCT and a 4.5‐year follow‐up. J Bone Miner Res 2011; 26: 35‐41.
- 37. Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70 528 patients from eight major vitamin D trials. J Clin Endocrinol Metab 2012; 97: 2670‐2681.
- 38. LeBoff MS, Chou SH, Ratliff KA, et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med 2022; 387: 299‐309.
- 39. Australian Skin and Skin Cancer Research Centre. Position statement balancing the harms and benefits of sun exposure. Brisbane: ASSC, 2023. https://www.assc.org.au/wp‐content/uploads/2023/01/Sun‐Exposure‐Summit‐PositionStatement_V1.9.pdf (viewed Sept 2024).
- 40. Iuliano S, Poon S, Robbins J, et al. Effect of dietary sources of calcium and protein on hip fractures and falls in older adults in residential care: cluster randomised controlled trial. BMJ 2021; 375: n2364.
- 41. Australian Government Department of Health and Ageing. Pharmaceutical Benefits Scheme. http://www.pbs.gov.au/ (viewed Nov 2024).
- 42. Ali MS, Berencsi K, Marinier K, et al. Comparative cardiovascular safety of strontium ranelate and bisphosphonates: a multi‐database study in 5 EU countries by the EU‐ADR Alliance. Osteoporos Int 2020; 31: 2425‐2438.
- 43. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014; 370: 412‐420.
- 44. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open‐label, phase 3 trial. Lancet 2017; 390: 1585‐1594.
- 45. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017; 377: 1417‐1427.
- 46. Australian Government Department of Health and Ageing. Pharmaceutical Benefits Advisory Committee (PBAC). Meeting outcomes, March 2023. https://www.pbs.gov.au/industry/listing/elements/pbac‐meetings/pbac‐outcomes/2023‐03/pbac‐web‐outcomes‐03‐2023‐v3.pdf (viewed Mar 2025).
- 47. Tsourdi E, Langdahl B, Cohen‐Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 2017; 105: 11‐17.
- 48. Lyu H, Zhao SS, Yoshida K, et al. Delayed denosumab injections and bone mineral density response: an electronic health record‐based study. J Clin Endocrinol Metab 2020; 105: 1435‐1444.
- 49. Everts‐Graber J, Reichenbach S, Ziswiler HR, et al. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res 2020; 35: 1207‐1215.
- 50. Sølling AS, Harsløf T, LangdahB. Treatment with zoledronate subsequent to denosumab in osteoporosis: a 2‐year randomized study. J Bone Miner Res 2021; 36: 1245‐1254.






Abstract
Introduction: This updated guideline replaces the previous Royal Australian College of General Practitioners and Osteoporosis Australia (now, Healthy Bones Australia) guideline from 2017. The accumulation of high quality evidence supporting improvements in clinical practice over the past five years, need for expert consensus and opinion, and new developments in pharmacological management of osteoporosis, especially the role of osteoanabolic therapies, prompted this update. The aim was to provide clear, evidence‐based recommendations to assist Australian general practitioners in managing patients over 50 years of age with poor bone health. However, it is useful for any health care professional caring for people with poor bone health and for health administrators and bureaucrats responsible for resource provision and allocation.
Main recommendations:
Changes in assessment and management as a result of the guideline: This guideline provides recommendations for the use of fracture risk assessment tools, particularly FRAX, for risk stratification, addresses the risk of rebound vertebral fracture following denosumab cessation, discusses removal of strontium as a therapy, clarifies “imminent” or “very high” fracture risk in patients and highlights the importance of calcium and vitamin D status, and the early use of osteoanabolic therapies. The full guideline is freely available at https://www.racgp.org.au/clinical‐resources/clinical‐guidelines/key‐racgp‐guidelines/view‐all‐racgp‐guidelines/osteoporosis/executive‐summary.