Randomised controlled trials (RCTs) support clinical practice by generating high quality evidence about the safety and effectiveness of medical interventions. However, RCTs are not required for the regulatory approval of new surgical procedures or devices.1 Surgical RCTs are subject to distinct practical challenges,2 and the quality of surgical trials has been criticised,3 although it appears to be improving.4 Few studies have directly compared the design features of surgical and non‐surgical trials, or examined differences between surgical clinical trials by specialty. We therefore determined the proportion of surgical clinical trials in Australia relative to all clinical trials and examined their characteristics, and compared surgical trial activity by specialty.
We reviewed all randomised and non‐randomised clinical trials registered with the Australian New Zealand Clinical Trials Registry (ANZCTR; https://anzctr.org.au) or ClinicalTrials.gov (https://clinicaltrials.gov) during 1 January 2010 – 29 February 2020 that included recruitment of adult participants (18 years or older) in Australia. We defined a surgical trial as one in which the intervention was delivered by surgeons with the aim of improving surgical outcomes for patients (further details: Supporting Information, methods). We used information in trial registration records to assess trial activity (number of trials, planned recruitment size), design (randomisation, masking), and industry involvement. For surgical trials, we compared these characteristics by specialty (cardio‐thoracic surgery; otolaryngology, head and neck surgery; general [including colorectal] surgery; neurosurgery; orthopaedic surgery; plastic and reconstructive surgery; urological surgery; vascular surgery; ophthalmology surgery; transplantation surgery). We characterised trial activity by specialty as the ratio of its proportion of all surgical trials to the proportion of in‐hospital surgical procedures for the specialty during 1 July 2010 – 30 June 2020, as recorded by the Australian Institute of Health and Welfare.5 If interventions or procedures could be performed in more than one specialty, we initially classified it under one specialty for the primary analysis, then remapped it to the alternative specialty in a sensitivity analysis (further details: Supporting Information, methods). Statistical analyses were performed in RStudio 2022.7.2.576. We did not seek formal ethics approval for our analysis of publicly available data.
Of 12 775 clinical trials with planned recruitment of adults in Australia registered during 2010–20, 435 were surgical trials (3.4%); 311 surgical (71%) and 8802 non‐surgical trials (72%) were RCTs, and industry involvement was recorded for 128 surgical (29%) and 5531 non‐surgical trials (45%) (Box 1). The annual number of surgical trial registrations rose from 24 in 2010 to 50 in 2019; in most years, the largest number of trials registered were for orthopaedic and general surgery (Supporting Information, figure 1). Clinical trials were identified for each of the ten surgical specialties, but the number and their characteristics varied widely (Supporting Information, table 1). The numbers of registrations were largest for orthopaedic surgery (142 trials, 33% of surgical trials; 7 092 232 procedures, 19% of surgical procedures; ratio, 1.71) and general surgery (97 trials, 22%; 9 804 429 procedures, 26%; ratio, 0.85). Trial activity relative to procedure number was highest for transplantation (ratio, 84.8) and cardio‐thoracic surgery (ratio, 3.47), and lowest for plastic (ratio, 0.42), urological (ratio, 0.42), and ophthalmology surgery (ratio, 0.49) (Box 2). In the sensitivity analysis, reclassification of trials and procedures that could be associated with two specialties changed the ratio for orthopaedic surgery from 1.71 to 1.42 and that for neurosurgery from 0.88 to 1.00 (Supporting Information, table 4). The relationship of the numbers of RCTs and procedures by specialty were similar to those for all surgical trials (Supporting Information, figure 2). The numbers and total planned recruitment size of surgical trials by specialty (excluding transplantation surgery) each differed from those predicted by the numbers of procedures (χ2 goodness‐of‐fit tests, P < 0.001).
Surgical trials comprised 3.4% of registered Australian clinical trials during 2010–20, while 23% of hospitalisations in Australia in 2022–23 were related to non‐obstetric surgery.6 The proportions of surgical and non‐surgical trials that used randomisation were similar, but the proportion that used masking was slightly smaller for surgical than non‐surgical RCTs (59% v 67%). Industry was involved in a smaller proportion of surgical trials than of non‐surgical trials, possibly reflecting differences in regulatory requirements for new devices and medicines.
Differences in the numbers of surgical trials by specialty did not reflect differences in the volume of surgical activity. Alternative explanations include differences in the use of new devices, research training, data collection and audit culture, trial infrastructure, academic appointments, and perceived need. The volume of surgical activity provides a denominator for comparisons of trial activity, but it does not provide information about the optimal number of surgical trials for a specialty.
As trial registry records rely on investigator input, our comparisons of trial characteristics will be affected if important details were omitted. For multinational trials, the planned recruitment size included overseas participants, but these trials were still relevant to Australian surgical practice. Hospital procedures data used to assess surgical activity did not include invasive procedures that can be performed by physicians or surgeons (eg, colonoscopies), which may have led to underestimation of surgical activity for some specialties. We examined trial registrations as a measure of the number of trials initiated, but not all registered trials are completed.7 Investigating how many surgical trials are completed and how they change practice would be valuable.
We report recent clinical trial activity by surgical specialty in Australia, and our findings could inform discussions of surgical research priorities and governance. The relatively low number of clinical trials in surgery suggests that strategies for supporting surgical trials are needed in Australia, including dedicated funding. The ANZCTR is a valuable public resource for monitoring the impact of such strategies.
Box 1 – Characteristics of registered clinical trials with planned recruitment of adults in Australia, 1 January 2010 – 29 February 2020, by trial type (surgical or non‐surgical)
|
Characteristic |
Surgical* |
Non‐surgical |
P † |
||||||||||||
|
|
|||||||||||||||
|
Clinical trials |
435 |
12 340 |
|
||||||||||||
|
Registry |
|
|
< 0.001 |
||||||||||||
|
ANZCTR |
376 (86%) |
8063 (65%) |
|
||||||||||||
|
ClinicalTrials.gov |
59 (14%) |
4277 (35%) |
|
||||||||||||
|
Purpose |
|
|
< 0.001 |
||||||||||||
|
Treatment |
371 (85%) |
9171 (74%) |
|
||||||||||||
|
Prevention |
49 (11%) |
1696 (14%) |
|
||||||||||||
|
Diagnosis |
9 (2%) |
450 (4%) |
|
||||||||||||
|
Other |
6 (1%) |
1023 (8%) |
|
||||||||||||
|
Recruitment |
|
|
< 0.001 |
||||||||||||
|
Australia only |
372 (86%) |
8607 (70%) |
|
||||||||||||
|
Australia and overseas |
63 (14%) |
3733 (30%) |
|
||||||||||||
|
Planned recruitment size |
|
|
0.001 |
||||||||||||
|
1–100 |
271 (62%) |
6981 (57%) |
|
||||||||||||
|
101–1000 |
154 (35%) |
4567 (37%) |
|
||||||||||||
|
More than 1000 |
10 (2%) |
792 (6%) |
|
||||||||||||
|
Randomisation |
|
|
0.99 |
||||||||||||
|
Yes |
311 (71%) |
8802 (72%) |
|
||||||||||||
|
No |
124 (29%) |
3496 (28%) |
|
||||||||||||
|
Missing information |
0 |
42 |
|
||||||||||||
|
Masking (for randomised trials) |
|
|
0.006 |
||||||||||||
|
Masked |
175 (59%) |
5650 (67%) |
|
||||||||||||
|
Open |
120 (41%) |
2755 (33%) |
|
||||||||||||
|
Missing information |
16 |
397 |
|
||||||||||||
|
Primary sponsor |
|
|
< 0.001 |
||||||||||||
|
Government |
14 (3%) |
410 (3%) |
|
||||||||||||
|
Industry |
82 (19%) |
4389 (36%) |
|
||||||||||||
|
Other |
339 (78%) |
7541 (61%) |
|
||||||||||||
|
Industry involvement |
|
|
< 0.001 |
||||||||||||
|
Yes |
128 (29%) |
5531 (45%) |
|
||||||||||||
|
No |
307 (71%) |
6809 (55%) |
|
||||||||||||
|
|
|||||||||||||||
|
ANZCTR = Australian New Zealand Clinical Trials Registry. * Excludes 319 trials (2.5%) with surgery‐related registered trial titles or intervention codes, but which did not meet our study criteria for surgical trials. † χ2 tests for difference in proportions. |
|||||||||||||||
Box 2 – Numbers of registered surgical trials by numbers of procedures for the corresponding surgical specialty*
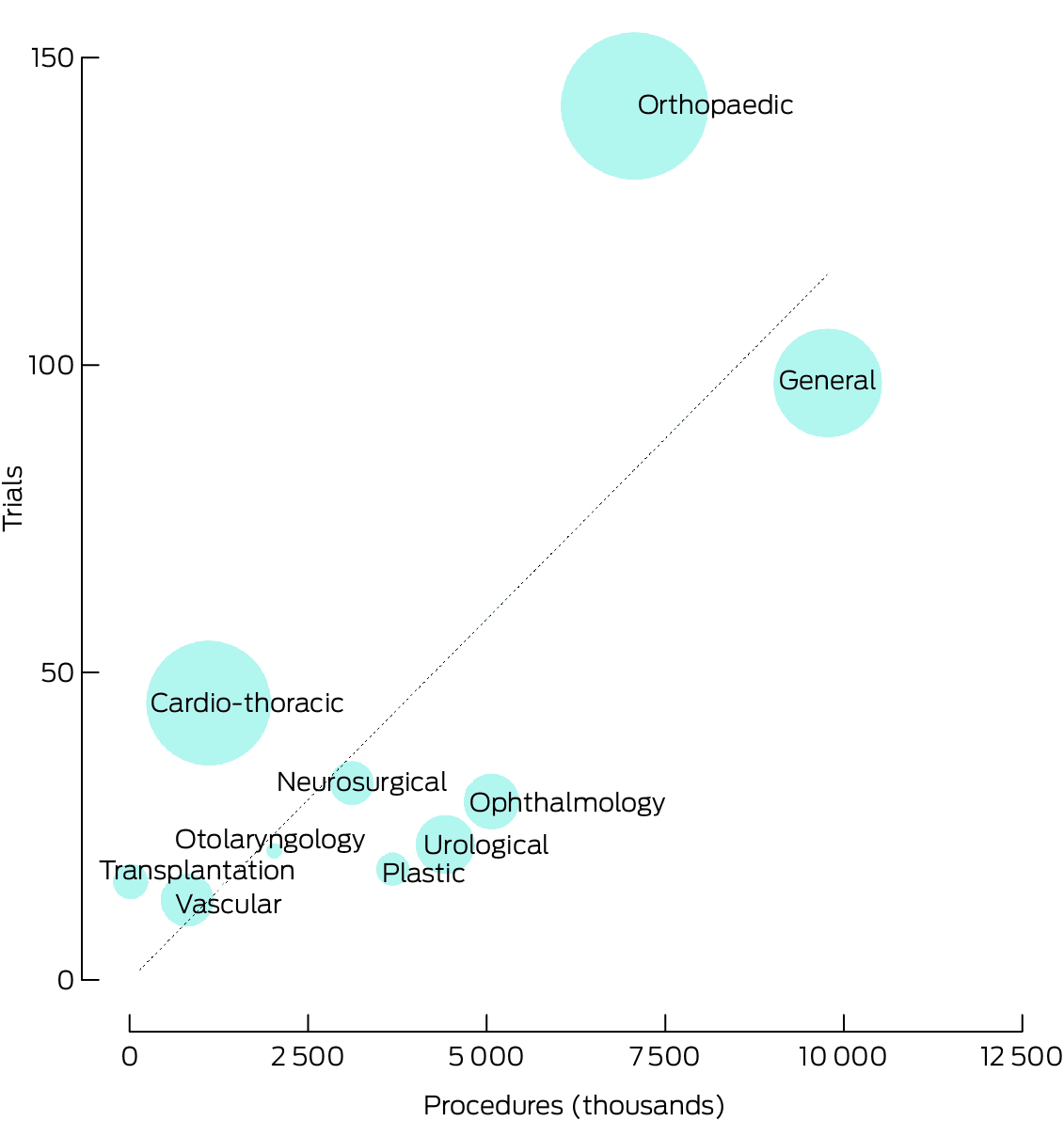
* The size of the circle is proportional to the planned recruitment numbers for the included trials. The diagonal line indicates the expected numbers of surgical trials were the proportions of trials by specialty the same as the proportions of surgical procedures. The data underlying this figure are included in the Supporting Information, tables 2 and 3.
Received 8 July 2024, accepted 11 October 2024
- 1. Therapeutic Goods Administration (Australian Department of Health and Aged Care). Medical devices: essential principles checklist. Updated 22 Aug 2024. https://www.tga.gov.au/resources/resource/forms/essential‐principles‐checklist‐medical‐devices (viewed Nov 2024).
- 2. McCulloch P, Taylor I, Sasako M, et al. Randomised trials in surgery: problems and possible solutions. BMJ 2002; 324: 1448‐1451.
- 3. Horton R. Surgical research or comic opera: questions, but few answers. Lancet 1996; 347: 984‐985.
- 4. Pronk AJM, Roelofs A, Flum DR, et al. Two decades of surgical randomized controlled trials: worldwide trends in volume and methodological quality. Br J Surg 2023; 110: 1300‐1308.
- 5. Australian Institute of Health and Welfare. Procedures data cubes. Updated 12 July 2023. https://www.aihw.gov.au/reports/hospitals/procedures‐data‐cubes/contents/summary (viewed Oct 2023).
- 6. Australian Institute of Health and Welfare. Admitted patients: Admitted patient care 2022–23. What procedures were performed? (here: tables S16.10, S16.15). Updated 11 Aug 2023. https://www.aihw.gov.au/reports‐data/myhospitals/intersection/activity/apc (viewed Mar 2024).
- 7. Wilson M, Seidler A, Aberoumand M, et al. Latest update of the clinical trials landscape in Australia 2006–2020. Sydney: Australian New Zealand Clinical Trials Registry, 2022. https://www.anzctr.org.au/docs/ClinicalTrialsInAustraliaUPDATE2006‐2020.pdf (viewed Oct 2024).





Data Sharing:
The data we analysed are freely available from the cited sources.
We thank the Australian New Zealand Clinical Trials Registry for their support in accessing and analysing clinical trials data for this study. We thank Richard G McGee (Campbelltown Hospital, South Western Sydney Local Health District) for his unpublished research into the frequency of published trials for frequently performed surgical procedures in Australia that informed our study design.
No relevant disclosures.