The known: The 2009 CKD‐EPI equation is used in Australia for estimating glomerular filtration rate without adjusting for ethnic background. The 2021 revision of the equation, without a race coefficient, has been widely adopted in the United States.
The new: In a large cohort of Australians aged 70 years or older, the proportion classified as having chronic kidney disease was markedly smaller with the 2021 (12%) than the 2009 CKD‐EPI equation (17%). The likelihood of reclassification was greater for women, and declined with age.
The implications: Were the CKD‐EPI 2021 equation adopted in Australia, the estimated prevalence of chronic kidney disease among generally healthy older people would be lower, potentially influencing decisions about treatment and specialist referrals.
The diagnosis and classification of chronic kidney disease (CKD) based on estimated glomerular filtration rate (eGFR) is important for secondary disease prevention, medication prescribing, treatment decisions, and timely specialist referrals.1 Reliable GFR estimation is particularly important for older adults, as they are more likely to be prescribed medications and referred to specialist care for other medical conditions.2 In Australia, GFR is estimated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation (CKD‐EPI2009) whenever serum creatinine is assessed. The recently published CKD‐EPI equation update (CKD‐EPI2021) does not adjust estimates for race.3 Recognising that race is a social and not a biological construct, CKD‐EPI2021 was validated using data from more than 4000 participants (mean age, 57.0 years; standard deviation, 17.4 years) in the United States, where its use is now recommended by the National Kidney Foundation4 and the American Society of Nephrology.5
Outside the United States, including in Australia, the CKD‐EPI2009 equation is typically used without adjusting for ethnic background, and the applicability and impact of introducing the revised equation has therefore been questioned.6,7 Medical care for people in certain population subgroups could be affected by using the new equation as a result of reclassification of their CKD status, including older adults. Investigation of the implications of the revised equation for older adults, particularly those with few other medical conditions, is limited. One recent study of adults of European and African descent aged 65 years or older found no clear benefit or harm in using CKD‐EPI2021 rather than CKD‐EPI2009.8 However, as CKD is more frequent in older people, any change in disease reporting could have important effects on diagnosis, referral patterns, and the management of risk factors. This is particularly important in Australia, given its ageing population and the increasing burden of CKD and related complications.9,10
Using the CKD‐EPI2021 could lead to higher eGFR estimates than the CKD‐EPI2009 and to reclassification of some people by Kidney Disease Improving Global Outcomes (KDIGO) CKD stage. We would expect that people reclassified to a less advanced CKD stage would generally be healthier than those not reclassified, and they would have similar risks of long term clinical outcomes; people reclassified to a more advanced CKD stage would be less healthy and at greater risk of negative long term outcomes. We therefore assessed the clinical impact on generally healthy older Australians of changing from CKD‐EPI2009 to CKD‐EPI2021.
Methods
In this article, we report the secondary analysis of data from the ASPirin in Reducing Events in the Elderly (ASPREE)11 and the ASPREE‐eXtension (ASPREE‐XT) studies.12 ASPREE (International Standard Randomized Controlled Trial Number Register, ISRCTN83772183; ClinicalTrials.gov, NCT01038583) was a double‐blind, randomised, placebo‐controlled, primary prevention trial conducted in Australia and the United States during 1 March 2010 – 12 June 2017; it examined whether daily 100 mg enteric‐coated aspirin extended disability‐free survival in healthy older people. In Australia, people aged 70 years or older and living in the community were recruited for participation during 1 March 2010 – 31 December 2014; people with hypertension were eligible for enrolment only if the condition was well controlled, and those with histories of cardiovascular disease, dementia, independence‐limiting physical disability, or other known five‐year life‐limiting illness were excluded. Participants were followed up for a median of 4.7 years (interquartile range, 3.6–5.7 years). ASPREE‐XT (ClinicalTrials.gov, NCT01038583) is a longitudinal observational study of ASPREE participants who consented to further follow‐up, commencing 1 February 2018 – 31 October 2019 (XT01) and with annual visits thereafter (XT02–XT06). In this study, we included data up to and including ASPREE‐XT02 for Australian participants only, and excluded those for whom baseline serum creatinine data were missing. We report our study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.13
Primary exposure
The primary exposure was baseline eGFR, calculated using the CKD‐EPI2009 and CKD‐EPI2021 equations (Supporting Information, supplementary methods). The KDIGO classifies CKD stage according to GFR (G1, 90 or greater; G2, 60–89; G3a, 45–59; G3b, 30–44; G4, 15–29; G5, less than 15 mL/min/1.73 m2; stages 3a to G5 indicate clinical CKD) and the urinary albumin‐to‐creatinine ratio (A1, less than 3; A2, 3–30; A3, more than 30 mg/mmol).14 We compared participants who were classified to different KDIGO CKD stages using the two equations with those whose classification was the same with both equations. Kidney function was assessed in each participant annually.
Outcomes
The primary outcome was disability‐free survival, with the primary endpoint defined as the first occurrence of death, dementia, or persistent physical disability.15 Secondary outcomes were major cardiovascular events (first occurrence of ischaemic stroke, myocardial infarction, or death from coronary heart disease, but excluding cardiac failure), hospitalisation with heart failure, death from any cause, and annual eGFR decline.
Other variables
Baseline variables included participant age, sex, education (less than twelve years, twelve years or more), dyslipidaemia, hypertension, diabetes, body mass index (BMI; less than 20, 20–24, 25–29, 30–34, 35 kg/m2 or more), smoking (currently, previously, never), alcohol use (currently, previously, never), polypharmacy, and urine albumin‐to‐creatinine ratio (log2 transformed). Dyslipidaemia was defined by the use of cholesterol‐lowering medications or a serum cholesterol level of 5.5 mmol/L or more. Hypertension was defined as mean blood pressure exceeding 140 mmHg (systolic) or 90 mmHg (diastolic) or by pharmacologic treatment for high blood pressure. Polypharmacy was defined as using five or more prescription medications.
Statistical analyses
Categorical variables are summarised as numbers and proportions, normally distributed continuous variables as means with standard deviations (SDs), and skewed continuous variables as medians with interquartile ranges (IQRs). The distributions of baseline eGFR with the two equations are presented in kernel density plots. For each equation, eGFR categories were cross‐tabulated to determine the distribution of participants and the proportions in each category reclassified by the newer equation.
We assessed changes in eGFR over time (per annual visit) according to each equation in linear mixed model. We assessed associations of variables with reclassification to a different eGFR category in three logistic regression analyses: an unadjusted model; a model adjusted for age and sex; and a model adjusted for age, sex, education, BMI, urine albumin‐to‐creatinine ratio, history of dyslipidaemia, diabetes, hypertension, smoking, alcohol use, and polypharmacy (fully adjusted model). We report unadjusted odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals (CIs).
We compared study outcomes for reclassified and other participants in three Cox proportional hazards regression models: an unadjusted model; a model adjusted for age and sex; and a model adjusted for age, sex, education, BMI, urine albumin‐to‐creatinine ratio, history of dyslipidaemia, diabetes, hypertension, smoking, alcohol consumption, and polypharmacy (fully adjusted model). Assumptions for proportional hazards were checked by visually checking survival curves for crossover and reviewing plotted Schoenfeld residuals for each study variable. We report unadjusted hazard ratios (HRs) and adjusted HRs (aHRs) with 95% CIs.
To assess whether changing from the 2009 to the 2021 CKD‐EPI equation would affect clinical outcomes by CKD stage, we undertook further analyses for three subgroups:
- participants with stage G3a CKD (eGFR, 45 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2 according to both equations);
- participants with stage G3b CKD (eGFR, 30 mL/min/1.73 m2 to less than 45 mL/min/1.73 m2 according to both equations); and
- participants with CKD stages G3a to G5 according to CKD‐EPI2009 (eGFR below 60 mL/min/1.73 m2) but not CKD‐EPI2021 (ie, eGFR with CKD‐EPI2021 of 60 mL/min/1.73 m2 or more).
Survival analyses (Cox proportional hazards regression models) compared the risk of study outcomes in these three subgroups with those for participants classified by both equations as not having CKD (eGFR 60 mL/min/1.73 m2 or more and urine albumin‐to‐creatinine ratio below 3 mg/mmol). The four subgroups were mutually exclusive. Statistical analyses were undertaken in R Studio 2023.12.01 (R Foundation for Statistical Computing).
Ethics approval
ASPREE was approved by multiple institutional review boards in the United States and Australia, and undertaken in accordance with the Declaration of Helsinki. The Monash University Human Research Ethics Committee approved the primary ASPREE study (MUHREC 2006000745) and the ASPREE‐XT secondary analyses (MUHREC 24743).
Results
Of the 16 703 Australian ASPREE trial participants, we excluded 459 for whom baseline serum creatinine measurements data were missing. Data for 16 244 people were included in our analysis (Supporting Information, figure 1), none of whom developed kidney failure (eGFR below 15 mL/min/1.73 m2) during the follow‐up period.
At baseline, the mean age of the 16 244 participants was 75.3 years (SD, 4.4 years); 8938 were women (55%). The median eGFR (CKD‐EPI2009) was 74 mL/min/1.73 m2 (IQR, 64–85 mL/min/1.73 m2), and the median urine albumin‐to‐creatinine ratio was 0.8 mg/mmol (IQR, 0.5–1.4 mg/mmol). A total of 12 190 participants had hypertension (75%), 11 034 had dyslipidaemia (68%), and 1601 had diabetes (10%) (Box 1).
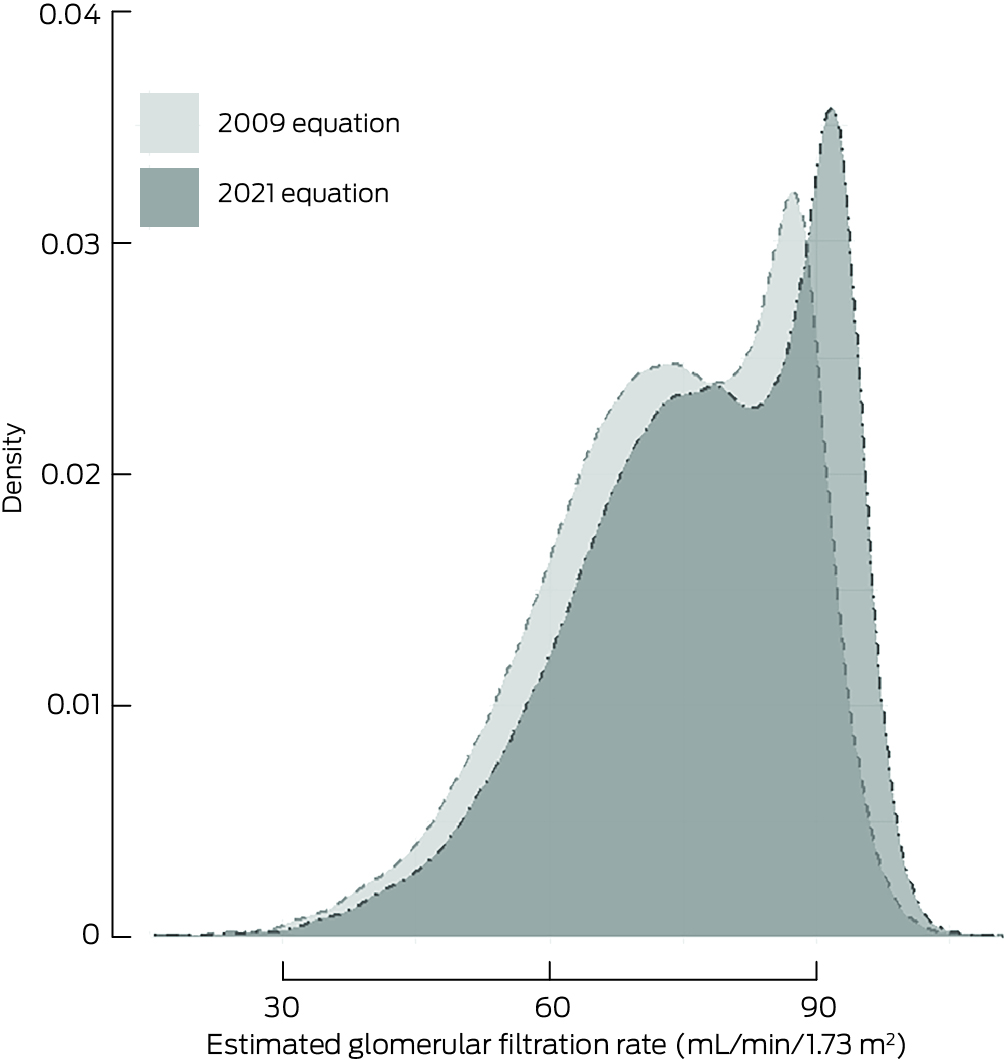
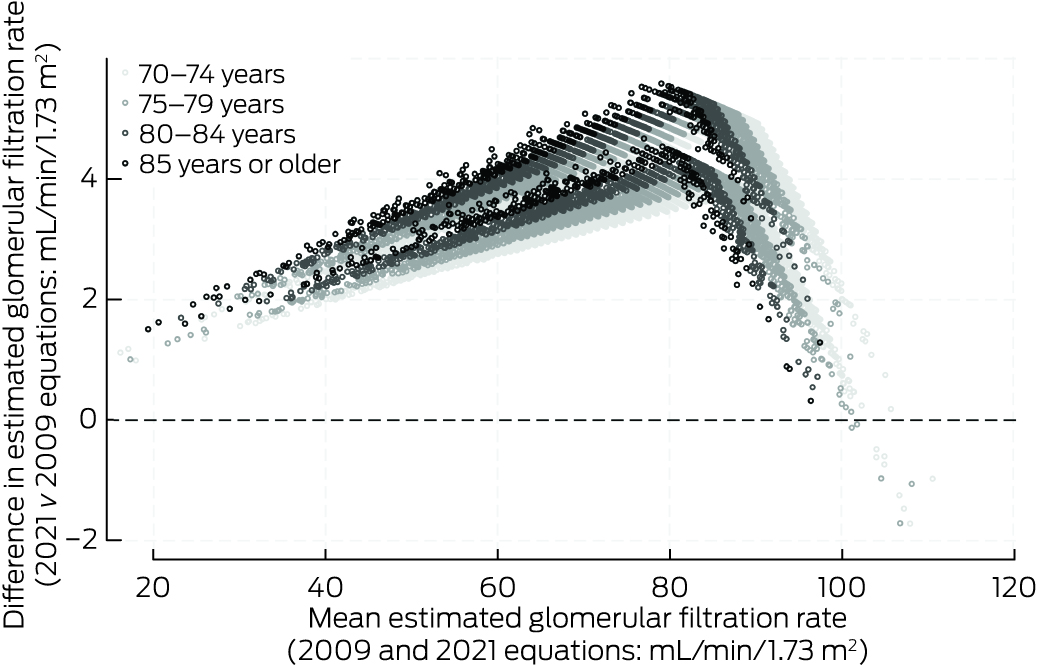
eGFR values were higher for most participants with CKD‐EPI2021 than with CKD‐EPI2009 (median difference, 3.8 mL/min/1.73 m2; IQR, 3.3–4.4 mL/min/1.73 m2) (Box 2). The differences were greatest for participants with CKD‐EPI2009 eGFR in the range 70–90 mL/min/1.73 m2 and for participants aged 80 years or older; CKD‐EPI2021 values were lower only for participants CKD‐EPI2009 eGFR values exceeding 100 mL/min/1.73 m2 (Box 3). The mean annual decline in eGFR during the follow‐up period was 1.07 mL/min/1.73 m2 (95% CI, 1.04–1.09 mL/min/1.73 m2) with CKD‐EPI2009, and 1.05 mL/min/1.73 m2 (95% CI, 1.03–1.08 mL/min/1.73 m2) with CKD‐EPI2021.
A total of 3274 participants (20%) were classified to different CKD stages by the two equations; in each case, CKD‐EPI2021 indicated a less advanced CKD stage. The proportion of participants with eGFR below 60 mL/min/1.73 m2 (clinical CKD) was 17% (2770 participants) with CKD‐EPI2009 and 12% (1994 participants) with CKD‐EPI2021 (Box 4).
Characteristics and outcomes for reclassified and other participants
In the fully adjusted model, the odds of reclassification declined with age (per year: aOR, 0.95; 95% CI, 0.94–0.96) and were higher for women (aOR, 1.51; 95% CI, 1.38–1.64). The likelihood of reclassification was not significantly higher for people with diabetes (aOR, 1.11; 95% CI, 0.97–1.28), dyslipidaemia (aOR, 0.94; 95% CI, 0.86–1.02), or polypharmacy (aOR, 1.04; 95% CI, 0.94–1.14) (Box 5).
Participants were followed up at a median of 6.5 years (IQR, 5.4–7.9 years); 2380 participants (15%) died or developed dementia or persistent physical disability during this period. The risk of reaching the disability‐free survival composite endpoint was lower for reclassified participants in the unadjusted model (HR, 0.81; 95% CI, 0.73–0.90), but not after adjustment for age and sex (aHR, 0.96; 95% CI, 0.86–1.07) (Box 6). The Kaplan–Meier curve for the fully adjusted model of disability‐free survival did not indicate a difference by reclassification status (Box 7).
The risks of long term health outcomes, including death (any cause; 1565 participants, 10%), major cardiovascular events (881 participants, 6%) and hospitalisation with heart failure (193 participants, 1%) were similar in fully adjusted models for participants who were reclassified or not reclassified (Box 6).
Reclassification of chronic kidney disease stage and long term outcomes
We compared the long term health outcomes for three subgroups of reclassified participants with those of participants classified as not having CKD according to both equations (11 474 participants for whom eGFR was at least 60 mL/min/1.73 m2) (Supporting Information, table 1). In the fully adjusted analyses, differences in the risks of disability‐free survival, all‐cause mortality, and hospitalisation with heart failure for the three groups were not statistically significant from those for the comparison group. The only significant difference with regard to major adverse cardiovascular events was that participants classified by both equations as having stage 3a CKD were at greater risk of a major adverse cardiovascular event than people without CKD (aHR, 1.34; 95% CI, 1.09–1.66) (Supporting Information, table 2 and figures 2 to 4).
Discussion
We assessed the potential clinical implications of using the CKD‐EPI2021 instead of the CKD‐EPI2009 for estimating GFR in a large group of generally healthy older Australians (70 years or older). We made three main findings. First, using the CKD‐EPI2021 shifted the eGFR distribution curve to higher values, resulting in the reclassification of 20% of people to less advanced CKD stages. Second, the likelihood of reclassification declined with age and was higher for women. Third, the long term health outcomes of participants who were reclassified did not differ from those of people who were not reclassified.
In our study, the proportion of older people classified as having CKD stages G3 to G5 was much smaller with CKD‐EPI2021 (12% of participants) than with CKD‐EPI2009 (17%), and eGFR values for most participants were higher (median difference, 3.8 mL/min/1.73 m2). Large European studies of adults (mean ages, 46–54 years) found that the prevalence of CKD was 0.7 to 1.3 percentage points lower with CKD‐EPI2021.3,16,17 The larger difference we found (five percentage points) is probably explained by the higher mean age of our cohort, an interpretation consistent with reports of larger differences when assessing older people with the CKD‐EPI2021 because of the age coefficient.3 As the development of CKD‐EPI2021 was validated in the United States in participants with a mean age of 57 years, its generalisability to older Australian adults was unclear. Although age‐specific eGFR equations developed for older adults have generally performed well in practice in Australia, the CKD‐EPI2009 is the default equation used by pathology laboratories, and it also accurately assesses kidney function in Indigenous Australians.18,19,20 According to our findings, changing to CKD‐EPI2021 in Australia would considerably reduce the estimated prevalence of clinical CKD in older adults.
Our findings contrast with those of an analysis of eight population‐based studies including more than 67 000 adult Asians which found that the proportion of people with diabetes was larger among those whose CKD stage was reclassified.21 Similarly, a recent longitudinal study found that reclassification of people aged 65 years or older to less advanced KDIGO CKD stages by CKD‐EPI2021 was more frequent for those with histories of diabetes or cardiovascular disease.17 The differences in locations and demographic characteristics probably account for these findings differing from ours.
People who are at greater risk of both cardiovascular disease and CKD progression could be inappropriately reclassified to less advanced CKD stages with CKD‐EPI2021.5 We found that most participants who were reclassified had mild to moderate CKD and were moved from stage G3a to G2 or from G2 to G1. As mild to moderate kidney disease (stage G3a or lower) is associated with lower risks of death and myocardial infarction than advanced kidney disease,22 the impact of reclassification on clinical decision‐making regarding these outcomes would be minimal. We did not find that the proportions of people with diabetes or dyslipidaemia were larger among people reclassified to less advanced CKD stages, and the risks of long term negative health outcomes were not affected by reclassification. Our findings differ from those of a study of 1.6 million Swedish adults which found that the risks of cardiovascular mortality, all‐cause mortality, and major cardiovascular events were higher for people reclassified to different CKD stages than for those who were not reclassified.17 However, most participants in the Swedish study were under 70 years of age. It is reassuring that our findings indicate that using CKD‐EPI2021 would not affect the prognosis of non‐kidney medical outcomes for older, generally well Australians.
The public health and primary care implications of using CKD‐EPI2021 for older adults must be carefully considered, as CKD is a major public health problem that primarily affects older Australians.23 We found that seven of 34 participants with stage G4 CKD (21%; eGFR, 15–29 mL/min/1.73 m2) using CKD‐EPI2009 were reclassified to stage G3b (eGFR, 30–44 mL/min/1.73 m2) by the CKD‐EPI2021 formula. Australian guidelines advise that people with eGFR values greater than 30 mL/min/1.73 m2, in the absence of other risk factors, can be appropriately managed in primary care.24 Moving to the 2021 equation could therefore reduce the number of referrals to specialist nephrology services.
The higher eGFR values using CKD‐EPI2021 could also affect when clinical disease is diagnosed in older adults. Whether an age‐adjusted CKD staging system should be used for older adults has been discussed for some time.25 Early diagnosis of CKD facilitates interventions for slowing disease progression, and eGFR thresholds are used to guide decisions.26 However, the definition of CKD in older adults has led to intense debate, as the physiological, age‐related decline in GFR is not taken into account by the KDIGO CKD definition.25,27 Using the 2021 equation could reduce the treatment burden for otherwise well older adults, provided treating practitioners are mindful of individual patient‐level factors.
Limitations
ASPREE provided a unique dataset based on a large group of generally healthy older, community‐dwelling adults with prospective longitudinal follow‐up, rich phenotypic data, and adjudicated health outcomes. However, about 85% of participants had preserved kidney function (eGFR greater than 60 mL/min/1.73 m2), which restricted our assessment of the implications of the change in equation for people with advanced stage CKD. As ASPREE enrolled generally healthy older adults, it is unclear whether these findings can be generalised to older adults who have other medical conditions with clinical indications for aspirin therapy. CKD‐EPI2009 and CKD‐EPI2021 were developed in United States populations of middle‐aged adults; equations for estimating GFR are most applicable to the populations in which they were developed. As we had no data on measured GFR or cystatin C‐based estimates of GFR, we could not compare the accuracy of CKD‐EPI2009 and CKD‐EPI2021 estimates. As no participants developed kidney failure during the follow‐up period, we could not assess it as an outcome. Older adults with CKD typically experience slower decline in kidney function than younger people, and they may die from associated conditions before kidney failure develops.28 Given the generally good health of the ASPREE participants, it is unsurprising that kidney failure was not an assessable outcome. It is unclear whether our findings can be generalised to Aboriginal and Torres Strait Islander Australians; Indigenous people were underrepresented in our sample, and CKD rates are higher among Indigenous than non‐Indigenous people.29 Similarly, it may be inappropriate to generalise our findings to older adults with other ethnic backgrounds underrepresented in the ASPREE cohort.
Conclusion
Implementing CKD‐EPI2021 in Australia would generally increase eGFR values compared with CKD‐EPI2009 and substantially reduce the estimated prevalence of CKD in older, generally healthy adults of predominantly European origin. Participants reclassified by the 2021 equation to less advanced GFR stages of CKD were not at greater risk of adverse clinical outcomes. These implications are important for health care in the community and for research should the revised equation be adopted in Australia.
Box 1 – Baseline characteristics of the Australian ASPREE participants, overall and according to whether the 2021 Chronic Kidney Disease–Epidemiology Collaboration equation reclassified their chronic kidney disease stage compared with the 2009 equation
|
Characteristic |
Overall |
Not reclassified |
Reclassified |
||||||||||||
|
|
|||||||||||||||
|
Participants |
16 244 |
12 970 |
3274 |
||||||||||||
|
Age (years), mean (SD) |
75.3 (4.4) |
75.6 (4.4) |
74.3 (3.9) |
||||||||||||
|
Sex (women) |
8938 (55%) |
6897 (53%) |
2041 (62%) |
||||||||||||
|
Ethnic background |
|
|
|
||||||||||||
|
Aboriginal or Torres Strait Islander |
11 (< 0.1%) |
11 (< 0.1%) |
0 |
||||||||||||
|
European |
16 019 (99%) |
12 777 (99%) |
3242 (99%) |
||||||||||||
|
Asian |
128 (0.8%) |
108 (0.8%) |
20 (0.6%) |
||||||||||||
|
Black |
4 (<0.1%) |
3 (<0.1%) |
1 (< 0.1%) |
||||||||||||
|
Other |
82 (0.4%) |
71 (0.6%) |
11 (0.3%) |
||||||||||||
|
Education |
|
|
|
||||||||||||
|
Less than 12 years |
8150 (50%) |
6485 (50%) |
1665 (51%) |
||||||||||||
|
12 years or more |
8093 (50%) |
6484 (50%) |
1609 (49%) |
||||||||||||
|
Drinks alcohol |
|
|
|
||||||||||||
|
Currently |
12 827 (79%) |
10 241 (79%) |
2586 (79%) |
||||||||||||
|
Formerly |
799 (5%) |
650 (5%) |
149 (5%) |
||||||||||||
|
Never |
2618 (16%) |
2079 (16%) |
539 (16%) |
||||||||||||
|
Smokes tobacco |
|
|
|
||||||||||||
|
Currently |
550 (3%) |
445 (3%) |
105 (3%) |
||||||||||||
|
Formerly |
6674 (41%) |
5370 (41%) |
1304 (40%) |
||||||||||||
|
Never |
9020 (56%) |
7155 (55%) |
1865 (57%) |
||||||||||||
|
Diabetes |
1601 (10%) |
1272 (10%) |
329 (10%) |
||||||||||||
|
Hypertension |
12 190 (75%) |
9776 (75%) |
2414 (74%) |
||||||||||||
|
Body mass index (kg/m2), median (IQR) |
27.4 (24.8–30.5) |
27.5 (24.9–30.5) |
27.4 (24.7–30.6) |
||||||||||||
|
Dyslipidaemia |
11 034 (68%) |
8780 (68%) |
2254 (69%) |
||||||||||||
|
Polypharmacy |
4239 (26%) |
3365 (26%) |
874 (27%) |
||||||||||||
|
CKD‐EPI2009 eGFR (mL/min/1.73 m2), median (IQR) |
75 (64–85) |
73 (65–81) |
87 (60–88) |
||||||||||||
|
Less than 15 |
0 |
0 |
0 |
||||||||||||
|
15–29 |
34 (0.2%) |
27 (0.2%) |
7 (0.2%) |
||||||||||||
|
30–44 |
460 (2.8%) |
307 (2.4%) |
153 (4.7%) |
||||||||||||
|
45–59 |
2276 (14%) |
1500 (12%) |
776 (24%) |
||||||||||||
|
60–89 |
12 092 (74%) |
9754 (75%) |
2338 (71%) |
||||||||||||
|
90 or greater |
1382 (8.5%) |
1382 (11%) |
0 |
||||||||||||
|
Urine albumin‐to‐creatinine ratio (mg/mmol), median (IQR) |
0.80 (0.50–1.40) |
0.80 (0.40–1.40) |
0.80 (0.50–1.30) |
||||||||||||
|
A1 (below 3) |
13 713 (89%) |
10 907 (89%) |
2806 (90%) |
||||||||||||
|
A2 (3–30) |
1597 (10%) |
1296 (11%) |
301 (9%) |
||||||||||||
|
A3 (more than 30) |
120 (1%) |
105 (1%) |
15 (1%) |
||||||||||||
|
|
|||||||||||||||
|
ASPREE = ASPirin in Reducing Events in the Elderly study; CKD‐EPI2009 = Chronic Kidney Disease–Epidemiology Collaboration 2009 equation; IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 2 – Distribution of baseline estimated glomerular filtration rate values using the 2009 and 2021 Chronic Kidney Disease–Epidemiology Collaboration equations: kernel density plot
Box 3 – Differences between estimated glomerular filtration rate values using 2009 and 2021 Chronic Kidney Disease–Epidemiology Collaboration equations, by mean baseline values (2009 and 2021 equations) and age group: Bland–Altman plot
Box 4 – Classification of participants by estimated glomerular filtration rate (eGFR) category using the 2009 and 2021 Chronic Kidney Disease–Epidemiology Collaboration equations
|
|
|
eGFR: CKD‐EPI2021 (mL/min/1.73 m2) |
|||||||||||||
|
eGFR: CKD‐EPI2009 (mL/min/1.73 m2) |
Participants (2009) |
90 or greater (G1) |
60–89 (G2) |
45–59 (G3a) |
30–44 (G3b) |
15–29 (G4) |
|||||||||
|
|
|||||||||||||||
|
Participants (2021) |
|
3720 |
10 530 |
1653 |
314 |
27 |
|||||||||
|
90 or greater (G1) |
1382 |
1382 (100%) |
— |
— |
— |
— |
|||||||||
|
60–89 (G2) |
12 092 |
2338 (19%) |
9754 (81%) |
— |
— |
— |
|||||||||
|
45–59 (G3a) |
2276 |
— |
776 (34%) |
1500 (66%) |
— |
— |
|||||||||
|
30–44 (G3b) |
460 |
— |
— |
153 (33%) |
307 (67%) |
— |
|||||||||
|
15–29 (G4) |
34 |
— |
— |
— |
7 (21%) |
27 (79%) |
|||||||||
|
|
|||||||||||||||
|
CKD‐EPI = Chronic Kidney Disease–Epidemiology Collaboration equation; eGFR = estimated glomerular filtration rate. Bold: reclassified participants. |
|||||||||||||||
Box 5 – ASPREE participant characteristics and odds of reclassification of chronic kidney disease glomerular filtration rate stage by the 2021 Chronic Kidney Disease–Epidemiology Collaboration equation: logistic regression analyses*
|
Characteristic |
Odds ratio (95% CI) |
Adjusted odds ratio† (95% CI) |
Adjusted odds ratio‡ (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Age, per year |
0.95 (0.94–0.96) |
0.95 (0.94–0.96) |
0.95 (0.94–0.96) |
||||||||||||
|
Sex (women) |
1.47 (1.35–1.59) |
1.49 (1.37–1.62) |
1.51 (1.38–1.64) |
||||||||||||
|
Education (12 years or more) |
0.97 (0.89–1.05) |
0.96 (0.89–1.04) |
0.95 (0.88–1.03) |
||||||||||||
|
Drinks alcohol |
|
|
|
||||||||||||
|
Currently |
1 |
1 |
1 |
||||||||||||
|
Formerly |
0.93 (0.77–1.12) |
0.97 (0.80–1.17) |
0.96 (0.80–1.16) |
||||||||||||
|
Never |
1.04 (0.93–1.16) |
0.99 (0.89–1.10) |
0.98 (0.88–1.10) |
||||||||||||
|
Smokes tobacco |
|
|
|
||||||||||||
|
Currently |
1 |
1 |
1 |
||||||||||||
|
Formerly |
1.01 (0.81–1.26) |
1.08 (0.86–1.35) |
1.11 (0.89–1.40) |
||||||||||||
|
Never |
1.09 (0.87–1.36) |
1.07 (0.86–1.34) |
1.11 (0.88–1.39) |
||||||||||||
|
Diabetes |
1.03 (0.90–1.17) |
1.09 (0.95–1.24) |
1.11 (0.97–1.28) |
||||||||||||
|
Hypertension |
0.92 (0.84–1.00) |
0.97 (0.88–1.06) |
0.97 (0.89–1.07) |
||||||||||||
|
Body mass index, per 1.0 kg/m2 |
0.99 (0.99–1.00) |
0.99 (0.98–1.00) |
0.99 (0.98–1.00) |
||||||||||||
|
Dyslipidaemia |
1.06 (0.97–1.15) |
0.94 (0.87–1.03) |
0.94 (0.86–1.02) |
||||||||||||
|
Polypharmacy |
1.04 (0.95–1.14) |
1.02 (0.93–1.12) |
1.04 (0.94–1.14) |
||||||||||||
|
Urine albumin‐to‐creatinine ratio, per 1.0 mg/mmol |
1.00 (0.98–1.03) |
1.01 (0.98–1.04) |
1.01 (0.98–1.04) |
||||||||||||
|
Urine albumin‐to‐creatinine ratio (mg/mmol), by category |
|
|
|
||||||||||||
|
A1 (below 3) |
1 |
1 |
1 |
||||||||||||
|
A2 (3–30) |
0.90 (0.79–1.03) |
0.97 (0.85–1.11) |
0.97 (0.85–1.01) |
||||||||||||
|
A3 (more than 30) |
0.52 (0.30–0.91) |
0.60 (0.34–1.05) |
0.60 (0.34–1.06) |
||||||||||||
|
|
|||||||||||||||
|
ASPREE = ASPirin in Reducing Events in the Elderly study; CI = confidence interval. * Excludes 814 participants (5.0%) for whom baseline urinary albumin measurement data were missing. † Adjusted for age, sex. ‡ Adjusted for age, sex, education, alcohol, smoking, diabetes, hypertension, body mass index, dyslipidaemia, polypharmacy, urinary albumin‐to‐creatinine ratio, baseline estimated glomerular filtration rate (Chronic Kidney Disease–Epidemiology Collaboration 2009 equation). |
|||||||||||||||
Box 6 – Long term health outcomes for ASPREE participants reclassified to a different chronic kidney disease glomerular filtration rate stage by the 2021 Chronic Kidney Disease–Epidemiology Collaboration equation: Cox proportional hazards regression analyses*
|
Outcome |
Hazard ratio (95% CI) |
Adjusted hazard ratio† (95% CI) |
Adjusted hazard ratio‡ (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Disability‐free survival§ |
0.81 (0.73–0.90) |
0.96 (0.86–1.07) |
0.94 (0.84–1.05) |
||||||||||||
|
All‐cause mortality |
0.78 (0.68–0.89) |
0.95 (0.83–1.09) |
0.90 (0.78–1.03) |
||||||||||||
|
Major adverse cardiovascular events |
0.85 (0.71–1.01) |
1.00 (0.84–1.19) |
0.94 (0.79–1.13) |
||||||||||||
|
Hospitalisations with heart failure |
0.78 (0.53–1.13) |
0.96 (0.66–1.41) |
1.00 (0.67–1.49) |
||||||||||||
|
|
|||||||||||||||
|
ASPREE = ASPirin in Reducing Events in the Elderly study; CI = confidence interval. * Median follow‐up: 6.5 years (interquartile range, 5.4–7.9 years); reference: participants who were not reclassified. Excludes 814 participants (5.0%) for whom baseline urinary albumin measurement data were missing. † Adjusted for age, sex. ‡ Adjusted for age, sex, education, alcohol, smoking, diabetes, hypertension, body mass index, dyslipidaemia, polypharmacy, urinary albumin‐to‐creatinine ratio, baseline estimated glomerular filtration rate (Chronic Kidney Disease–Epidemiology Collaboration 2009 equation). § Endpoint: first occurrence of death, dementia, or persistent physical disability. |
|||||||||||||||
Box 7 – Disability‐free survival for ASPREE participants, by whether the 2021 Chronic Kidney Disease–Epidemiology Collaboration equation reclassified their chronic kidney disease stage compared with the 2009 equation: Kaplan–Meier analysis*
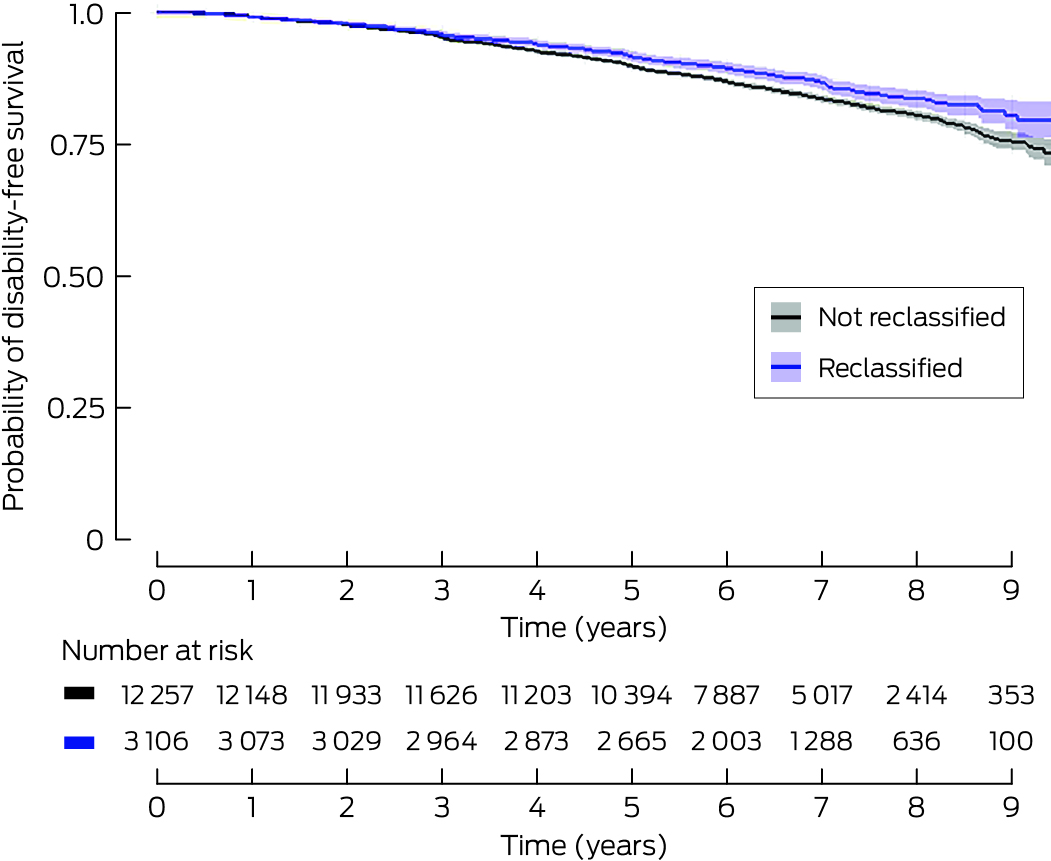
* Excludes 814 participants (5.0%) for whom baseline urinary albumin measurement data were missing. Adjusted for age, sex, education, alcohol, smoking, diabetes, hypertension, body mass index, dyslipidaemia, polypharmacy, urinary albumin‐to‐creatinine ratio, baseline estimated glomerular filtration rate (Chronic Kidney Disease–Epidemiology Collaboration 2009 equation). Reclassified v not re‐classified: P = 0.25.
Received 12 February 2024, accepted 8 July 2024
- Elisa K Bongetti1,2
- Rory Wolfe2
- James B Wetmore3
- Anne M Murray4
- Robyn L Woods2
- Michelle A Fravel5
- Mark R Nelson6
- Nigel P Stocks7
- Suzanne G Orchard2
- Kevan R Polkinghorne1,2
- 1 Monash Medical Centre, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 Hennepin Healthcare, Minneapolis, MN, United States of America
- 4 Berman Center for Outcomes and Clinical Research, Hennepin Healthcare Research Institute, Minneapolis, MN, United States of America
- 5 Carver College of Medicine, the University of Iowa, Iowa City, IA, United States of America
- 6 Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS
- 7 The University of Adelaide, Adelaide, SA
Data Sharing:
The data from the ASPREE randomised trial cohort underlying the reported study are available for sharing with researchers upon application at https://ams.aspree.org/public/request‐data/access‐aspree‐data.
The ASPREE study was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824), the National Health and Medical Research Council (NHMRC; 334047 and 1127060), Monash University, and the Victorian Cancer Agency. Elisa Bongetti is supported by an Australian government Research Training Program scholarship. The funding sources had no role in the planning, writing or publication of the research paper. They had no role in the study design, data collection, analysis, or interpretation of results.
No relevant disclosures.
- 1. Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end‐stage kidney disease. Cochrane Database of Syst Rev 2014; 18: CD007333.
- 2. Secora A, Alexander GC, Ballew SH, et al. Kidney function, polypharmacy, and potentially inappropriate medication use in a community‐based cohort of older adults. Drugs Aging 2018; 35: 735‐750.
- 3. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine‐ and cystatin C‐based equations to estimate GFR without race. N Engl J Med 2021; 385: 1737‐1749.
- 4. Miller WG, Kaufman HW, Levey AS, et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD‐EPI 2021 race‐free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin Chem 2022; 68: 511‐520.
- 5. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF‐ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis 2022; 79: 268‐288.
- 6. Gansevoort RT, Anders HJ, Cozzolino M, et al. What should European nephrology do with the new CKD‐EPI equation? Nephrol Dial Transplant 2023; 38: 1‐6.
- 7. Pierre CC, Greene DN, Marzinke M, et al. Refitting estimated glomerular filtration rate equations to find a fit for all. Association for Diagnostics and Laboratory Medicine, 1 July 2023. https://www.myadlm.org/cln/articles/2023/julaug/refitting‐estimated‐glomerular‐filtration‐rate‐equations‐to‐find‐a‐fit‐for‐all (viewed Sept 2023).
- 8. Delanaye P, Vidal‐Petiot E, Björk J, et al. Performance of creatinine‐based equations to estimate glomerular filtration rate in White and Black populations in Europe, Brazil and Africa. Nephrol Dial Transplant 2023; 38: 106‐118.
- 9. Sims J. Our ageing population: insights from the 2016 census. Australas J Ageing. 2017; 36: 176.
- 10. Australia and New Zealand Dialysis and Transplant Registry. Incidence of kidney failure with replacement therapy. In: ANZDATA 44th annual report 2021 (data to 2020). Updated 1 June 2022. https://www.anzdata.org.au/wp‐content/uploads/2023/09/ANZDATA_AR‐2022‐23_Chapter‐1_F4.pdf (viewed Nov 2024).
- 11. ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013; 36: 555‐564.
- 12. Ernst ME, Broder JC, Wolfe R, et al; ASPREE Investigator Group. Health characteristics and aspirin use in participants at the baseline of the ASPirin in Reducing Events in the Elderly – eXTension (ASPREE‐XT) observational study. Contemp Clin Trials 2023; 130: 107231.
- 13. von Elm E, Altman DG, Egger M, Vandenbroucke JP, et al; STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806‐808.
- 14. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease guideline development work group members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825.
- 15. McNeil JJ, Woods RL, Nelson MR, et al; ASPREE Investigator Group. Effect of aspirin on disability‐free survival in the healthy elderly. N Engl J Med 2018; 379: 1499‐1508.
- 16. Escribano‐Serrano J, Jiménez‐Varo E, Escribano‐Cobalea M, et al; Grupo de Estudio de Riesgo Vascular Alcalá (GERVA). Is the use of the new Chronic Kidney Disease Epidemiology Consortium (CKD‐EPI 2021) formula appropriate for the Spanish population? Rev Clin Esp (Barc) 2023; 223: 144‐153.
- 17. Fu EL, Coresh J, Grams ME, et al. Removing race from the CKD‐EPI equation and its impact on prognosis in a predominantly White European population. Nephrol Dial Transplant 2023; 38: 119‐128.
- 18. Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798‐806.
- 19. Delanaye P, Cavalier E, Pottel H, Stehlé T. New and old GFR equations: a European perspective. Clin Kidney J 2023; 16: 1375‐1383.
- 20. Maple‐Brown LJ, Hughes JT, Lawton PD, et al. Accurate assessment of kidney function in Indigenous Australians: the estimated GFR study. Am J Kidney Dis 2012; 60: 680‐682.
- 21. Betzler BK, Sultana R, He F, et al. Impact of Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) GFR estimating equations on CKD prevalence and classification among Asians. Front Med (Lausanne) 2022; 9: 957437.
- 22. Kühn A, van der Giet M, Kuhlmann MK, et al. Kidney function as risk factor and predictor of cardiovascular outcomes and mortality among older adults. Am J Kidney Dis 2021; 77: 386‐396.e1.
- 23. Australian Bureau of Statistics. Australian health survey: biomedical results for chronic diseases, 2011–12. 5 Aug 2013. https://www.abs.gov.au/statistics/health/health‐conditions‐and‐risks/australian‐health‐survey‐biomedical‐results‐chronic‐diseases/latest‐release (viewed Sept 2023).
- 24. Johnson D. When to refer for specialist renal care. CARI guidelines, Mar 2012. https://www.cariguidelines.org/guidelines/chronic‐kidney‐disease/early‐chronic‐kidney‐disease/when‐to‐refer‐for‐specialist‐renal‐care (viewed Jan 2024).
- 25. Glassock R, Delanaye P, El Nahas M. An age‐calibrated classification of chronic kidney disease. JAMA 2015; 314: 559‐560.
- 26. Johnson DW. Evidence‐based guide to slowing the progression of early renal insufficiency. Intern Med J 2004; 34: 50‐57.
- 27. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024; 105: S117–S314.
- 28. O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758‐2765.
- 29. Hoy WE, Mott SA, McDonald SP. An expanded nationwide view of chronic kidney disease in Aboriginal Australians. Nephrology 2016; 21: 916‐922.





Abstract
Objectives: To assess the clinical impact on generally healthy older Australians of changing from the 2009 CKD‐EPI (CKD‐EPI2009) to the 2021 CKD‐EPI (CKD‐EPI2021) equation for calculating the estimated glomerular filtration rate (eGFR).
Study design: Secondary analysis of data from the prospective ASPirin in Reducing events in the Elderly (ASPREE) cohort study.
Setting, participants: Australians aged 70 years or older living in the community and without life‐limiting medical conditions, recruited 1 March 2010 – 31 December 2014 for the ASPREE trial.
Main outcome measures: Baseline characteristics and long term health outcomes for participants classified to different chronic kidney disease (CKD) stages by CKD‐EPI2021 and CKD‐EPI2009, and for those classified to the same CKD stage by both equations.
Results: Complete data were available for 16 244 Australian ASPREE trial participants. At baseline, their mean age was 75.3 years (standard deviation, 4.4 years), and 8938 were women (55%); the median eGFR (CKD‐EPI2009) was 74 mL/min/1.73 m2 (interquartile range [IQR], 64–85 mL/min/1.73 m2), the median urine albumin‐to‐creatinine ratio 0.8 mg/mmol (IQR, 0.5–1.4 mg/mmol). eGFR values were higher for most participants with CKD‐EPI2021 than with CKD‐EPI2009 (median difference, 3.8 mL/min/1.73 m2; IQR, 3.3–4.4 mL/min/1.73 m2), and 3274 participants (20%) were classified to less advanced CKD stages by CKD‐EPI2021. The proportion of participants with eGFR values below 60 mL/min/1.73 m2 (clinical CKD) was 17% (2770 participants) with CKD‐EPI2009 and 12% (1994 participants) with CKD‐EPI2021. Participants were followed up at a median of 6.5 years (IQR, 5.4–7.9 years); the risks of reaching the disability‐free survival composite endpoint (adjusted hazard ratio [aHR], 0.94; 95% confidence interval [CI], 0.84–1.05), all‐cause mortality (aHR, 0.90; 95% CI, 0.78–1.03), major cardiac events (aHR, 0.94; 95% CI, 0.79–1.13), and hospitalisations with heart failure (aHR, 1.00; 95% CI, 0.67–1.49) were each similar for participants reclassified or not reclassified by CKD‐EPI2021.
Conclusions: Using CKD‐EPI2021 would yield higher eGFR values than the CKD‐EPI2009, substantially reducing the proportion of older Australian adults classified as having CKD, without any overall difference in long term health outcomes for people reclassified to less advanced CKD stages. Using the CKD‐EPI2021 could markedly reduce the number of referrals of generally healthy older adults to specialist nephrology services.