Buruli ulcer is a neglected tropical disease, as recognised by the World Health Organization (WHO).1 In the past decade, research has continued to expand our understanding of Buruli ulcer as the incidence has increased in Australia.2 This consensus statement aims to provide a pragmatic and evidence‐based update to guide clinicians in the diagnosis and management of Buruli ulcer in Australia.
Buruli ulcer is caused by the environmental bacterium Mycobacterium ulcerans, which infects the skin and subcutaneous tissue, with a preference for cooler growth conditions.3 M. ulcerans produces a potent necrotising and immunosuppressive toxin, mycolactone, which is responsible for its unique pathology.4 Local research has revealed that transmission of M. ulcerans in Australia is a complex One Health interaction involving mosquitoes, native possums (mainly ringtail), the environment (soil characteristics, fauna, infrastructure) and humans (behaviour, health and vaccination status).5,6,7,8,9
The incubation period between infection and disease manifestation is about five months (range, 1–9 months).10 Therefore, while transmission usually occurs during the warmer months, disease incidence peaks in winter.11 Most Buruli ulcer lesions occur on body regions exposed to the environment, typically the legs and arms.11 Early lesions include nodules, plaques, or areas of localised erythema and/or oedema; these may be initially interpreted as arthropod bites. Lesions will usually progress slowly over weeks and develop into ulcers, although around 10% will be non‐ulcerative at diagnosis (Box 1).12 As the organism multiplies in the subcutaneous tissue, the ulcer typically develops a characteristic undermined edge with surrounding induration. It is classically painless, although the oedematous/cellulitic form may rarely present more acutely with pain and fever.13
Epidemiology
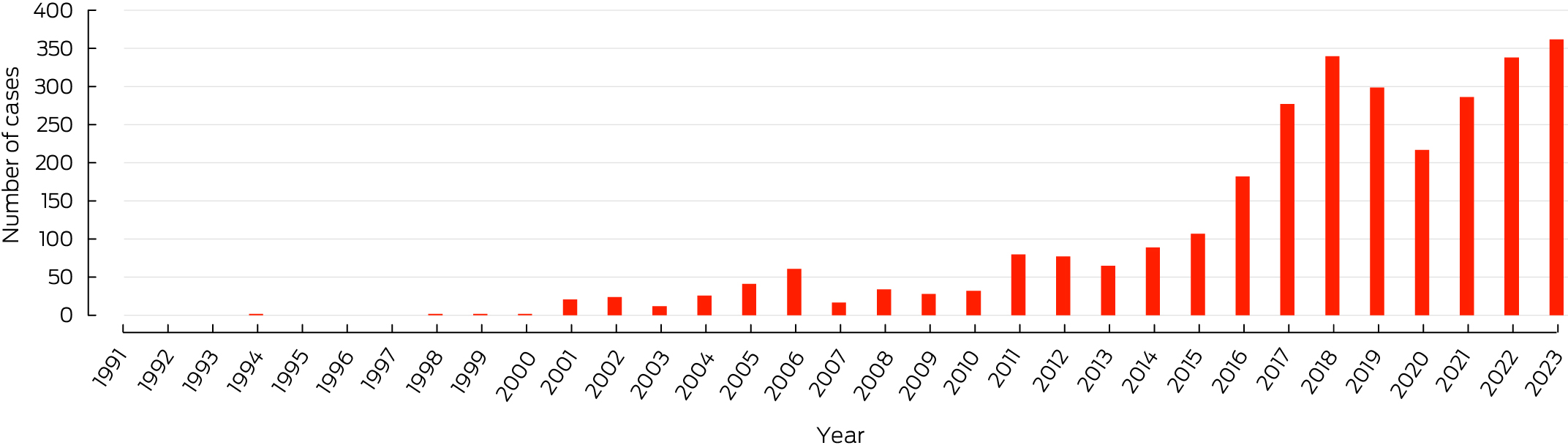
Outbreaks in Australia have largely been focal and predominantly occurred in coastal regions of the Mornington and Bellarine Peninsulas in Victoria, and near Cairns in far‐north Queensland.14,15 The epidemiology has shifted in recent years to include suburbs of Melbourne in the inner north‐west and Geelong.16 The incidence in Victoria has continued to increase, with a record number of new diagnoses (362 cases) reported in 2023 (Box 2).17 Local transmission has also recently been reported in Bateman's Bay, New South Wales, which is the closest coastal town to Canberra, Australian Capital Territory (Box 3).18 In Australia, Buruli ulcer was first notifiable in the Northern Territory in 1994,19 then subsequently in Victoria in 2004 and Queensland in 2005. Cases of Buruli ulcer identified in other states or territories should be discussed with relevant health authorities, even if not notifiable, and there is now equipoise for making Buruli ulcer nationally notifiable. The NT is the only jurisdiction that reports if individuals with Buruli ulcer identify as Aboriginal and Torres Strait Islander people.
Disease incidence is not restricted by age, sex, ethnicity, socio‐economic status, or medical comorbid conditions, although people with diabetes are at increased risk.5 As incidence rises and new, highly populated regions become endemic, clinicians and members of the community should have a high index of suspicion to ensure timely diagnosis and treatment.
Methods
An update of the 2014 consensus guidelines20 was undertaken by select adult and paediatric infectious diseases physicians, public health practitioners, primary health care providers, and wound care experts with experience in Buruli ulcer. An initial draft document based on evidence from current research and recent clinical experience was prepared by DB, SM, VC, MB and PJ before review by all authors. In July 2024, a consensus group discussion meeting was then held via hybrid teleconference, with discussion agenda items raised from the review of the draft manuscript. The consensus meeting was chaired by SM. Scientific evidence was discussed, including strengths and limitations of the available evidence. In the absence of evidence, clinical experience was also discussed. Consensus was defined by universal agreement among the group, reached by discussion. The meeting was recorded and the text transcribed to ensure suggestions were captured. The consensus statement document was then refined, peer reviewed, and endorsed by the Australasian Society for Infectious Diseases. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (https://www.gradeworkinggroup.org) was used to classify the quality of published evidence. Very low or low quality of evidence (observational case series/expert opinion) is used throughout this consensus guideline, except where specific references are cited.
Clinical consensus statement
Diagnosis
Use of IS2404 polymerase chain reaction (PCR) testing directly from ulcer swabs remains the recommended method to diagnose Buruli ulcer given the ease, speed, accuracy and availability of testing.21 The swab should circle the undermined edge of the ulcer, and clinical material from the lesion should be visible on the swab surface.22 Using sterile saline to moisten the wound or the swab to loosen biological material may improve sampling if required. False negative PCR results are not uncommon, especially for early lesions or in children.22 Thus, for non‐ulcerative or pre‐ulcerative lesions (eg, oedematous, plaques or nodules), or suspicious lesions with a negative PCR swab result, a skin biopsy from the centre of non‐ulcerated lesions, or from the margin of ulcerated lesions, should be undertaken for PCR testing and to evaluate alternative diagnoses (eg, malignancy). Fresh tissue specimens sent for microbiological investigation should not be placed in preservative; however, placing the specimen in saline soaked gauze (or adding drops of saline to the tissue specimen) will prevent dehydration. Diagnosis from mycobacterial culture is less sensitive than PCR and results can take up to 12 weeks due to the organism's slow growth.21 We recommend undertaking a PCR in all cases, but when this is not possible, a probable diagnosis of Buruli ulcer can be made without PCR if a clinically consistent lesion has acid‐fast bacilli on microscopy and patient exposure to an endemic region.23 Histopathological features of Buruli ulcer depend on the clinical stage of disease.24 Necrosis of subcutaneous tissues and dermal collagen accompanied by minimal inflammation and acid‐fast bacilli are considered the most reliable histopathological features for the diagnosis of Buruli ulcer.25 Epidermal hyperplasia and chronic inflammation with formation of granulomas is more frequent in ulcers compared with pre‐ulcerative lesions.25
Antibiotics
Most cases of Buruli ulcer are now managed without surgery, after trials reported most patients achieved wound healing with appropriate antibiotic therapy.26 Antibiotics targeting the usual organisms that cause skin and soft tissue infection are not effective against M. ulcerans, but may be associated with temporary improvement, which may mislead clinicians. In the absence of clinical features of bacterial superinfection, antibiotic treatment of colonising bacteria is not recommended. A dual antibiotic combination for Buruli ulcer should be used to increase treatment effectiveness and reduce the risk of antibiotic resistance, although resistance to first line antibiotics has not been reported in Australia. Regimens using an injectable agent are no longer recommended.27 Routine antibiotic susceptibility testing is currently unavailable in Australia, although it remains a research priority.
Local observational studies have shown that primary rifampicin‐based regimens are highly effective.28 Current WHO guidelines recommend combining rifampicin with clarithromycin.29 We recommend rifampicin‐containing combination oral antibiotic therapy for eight weeks as a first line treatment for most patients with Buruli ulcer (GRADE: high quality evidence). Recommended doses are rifampicin 10 mg/kg per day up to 600 mg daily, plus any of clarithromycin 7.5 mg/kg twice daily (up to 500 mg every 12 hours), moxifloxacin 400 mg once daily or ciprofloxacin 500 mg every 12 hours. The choice between using clarithromycin or a fluoroquinolone (as the initial companion drug to rifampicin) is individualised, with considerations including the patient's comorbid conditions, drug interactions and tolerability. Clarithromycin is the preferred macrolide for treatment of M. ulcerans infection based on the available evidence; although azithromycin may also have activity,30 there is minimal experience with its use in adults with Buruli ulcer.
If rifampicin is contraindicated or not tolerated, we recommend clarithromycin combined with a fluoroquinolone antibiotic for eight weeks.31 In pregnancy, the combination of rifampicin and clarithromycin is recommended.29 Since November 2020, rifampicin has been available on the Australian Pharmaceutical Benefits Scheme for the management of Buruli ulcer, which has reduced the cost and improved treatment access.
Observational evidence suggests a shorter duration of antibiotic therapy of six weeks may be reasonable if immunocompetent adults have small (< 3 cm in diameter) ulcerated lesions32 or experience severe adverse effects, especially if combined with surgery.33 Treatment failure may be increased in patients weighing more than 90 kg, who are immunocompromised, taking rifampicin–clarithromycin combinations, and who are male.34 For patients with a combination of risk factors, a shortened course of antibiotics is best avoided, and a rifampicin and fluoroquinolone combination may be preferred. Monotherapy is avoided due to the risk of treatment‐emergent resistance.35 There is insufficient evidence and limited experience with using increased antibiotic doses for individuals who are overweight or obese. There is limited evidence to guide antibiotic therapy duration in significantly immunocompromised patients, although prolonging therapy in individuals with M. ulcerans and human immunodeficiency virus co‐infection is not recommended by the WHO.29 General principles include reducing immunosuppression if safe and feasible, and extending the duration of antibiotics in selected cases, in consultation with an experienced infectious disease specialist and their treating physician.36
Drug‐related toxicity occurs in about 20% of patients treated for Buruli ulcer, with an increased risk in those who are older than 65 years,37 female patients, and those with renal dysfunction.38 Common adverse effects include gastrointestinal intolerance, dysgeusia from clarithromycin, pharmacokinetic interactions, and drug‐induced liver injury. Drug hypersensitivity and neuropsychiatric disturbances occur less commonly. Full blood examination, and hepatic and renal function should be checked at baseline. Consider repeating these investigations periodically, particularly if there is any additional risk (eg, pre‐existing liver or renal disease, viral hepatitis, alcohol misuse, risk of cumulative toxicity with other medication). Patients should be instructed to avoid hepatotoxins during antibiotic treatment, which includes moderating alcohol. A risk of tendinitis is associated with prolonged fluoroquinolone use, particularly in older patients or those receiving corticosteroid treatment, thus patients should be instructed to report the development of tendon pain. Clarithromycin and fluoroquinolones can prolong the cardiac QTc interval; therefore, a baseline electrocardiogram should be performed and repeated after two weeks if the patient is at increased risk (eg, pre‐existing cardiac disease, borderline prolongation of QTc interval at baseline, a combination of QT prolonging medication). Clarithromycin and ciprofloxacin doses should be renally adjusted. Pharmacokinetic interactions are particularly common with rifampicin and can also occur with clarithromycin. All prescription and non‐prescription medications should be checked for drug interactions, and patients must be warned to check with their health practitioner when commencing new medication. Alternative contraception should be recommended for women using the oral contraceptive pill or progesterone implant and rifampicin; intrauterine devices which release levonorgestrel remain an effective form of contraception.39
Healing of Buruli ulcer lesions is much slower than expected for more common skin infections that respond to more familiar antibiotics and can be associated with significant necrosis (Box 1). Patients should be educated that ulcers typically enlarge with treatment and will not have healed when antibiotic therapy is ceased.40 The median duration of healing is 4.5 months in Australia, although this depends on the lesion size at antibiotic commencement (three months if < 2 cm, four months if 2–4 cm, and six months if > 4 cm in diameter), and is longer for oedematous lesions and in people aged 65 years or over. Prolonged healing may lead to significant expense and inconvenience from dressings and regular medical appointments, which may lead to time off work or school and significant patient dissatisfaction.29 Highly experienced clinicians are available to provide phone or telehealth support for clinically challenging cases.41
Surgery
Aggressive surgery is no longer recommended for the treatment of Buruli ulcer; however, there is still an important role for surgery in specific situations.42 A close working relationship between surgical and infectious diseases teams is essential to determine optimal treatment. Surgical approaches include:
- Debridement. Removal of necrotic or inflamed tissue in the wound base and edges to expedite wound healing and prevent deformity or scarring in lesions with significant necrosis. This should be as conservative as practicable and, if needed, may be repeated to remove new areas of necrosis or liquefied subcutaneous fat. Surgery can be performed after antibiotic treatment has concluded without affecting treatment success rates.43 Surgical debridement to remove the inflammatory stimulus from necrotic tissue in severe paradoxical reactions may lead to reduced tissue loss and improved healing times.
- Conservative excision. Narrow excision of macroscopically involved tissue may be used to expedite wound healing, to facilitate a shortened antibiotic duration if there are issues with tolerance, or to electively shorten the antibiotic treatment duration for small lesions (four weeks).44 Generally, this is done after four weeks of antibiotic therapy and only if direct closure can be achieved to avoid unnecessary cost, complexity and morbidity of reconstructive surgery with split skin grafts or vascularised flaps.
- Wide excision. Curative excisional surgery with wide margins can be considered if antibiotics are contraindicated, declined, or ceased early; usually for lesions whose size and position allows direct wound closure without the need for reconstructive surgery. However, there is a risk of disease relapse, either locally or distantly, which is increased if histological margins of the excised tissue include visible bacteria or active inflammation, if the patient is immunosuppressed, or the lesion had been present for more than 75 days before diagnosis.45
- Curette. For small lesions, a curette under local anaesthetic of only macroscopically involved necrotic tissue may allow a reduced antibiotic duration of four weeks.
Rarely is extensive reconstructive surgery required to repair large defects or hasten wound closure. Simple debridement can help secondary healing, which usually follows reasonably rapidly after the procedure. Despite some exceptions, skin grafting or vascularised tissue flaps should not be used for defect closure, but may be required to improve function or cosmesis at a later stage. If performed, such surgery should only be undertaken after antibiotic completion and after the wound is healed or almost healed, or there is an increased risk of failure to heal.
Paradoxical reaction
About one in five patients treated with antibiotics will develop a paradoxical reaction, also known as an immune reconstitution inflammatory reaction.46 Clinically, these are associated with increasing induration, ulceration, pain, wound discharge or onset of a new lesion after initial improvement. New lesions may occur during or after antibiotic treatment, at local or distant sites,46 and delay wound healing by two months on average.40 Paradoxical reactions are believed to be caused by reversal of local immunosuppression mediated by mycolactone against mycobacterial antigens. Risk factors in Australia include oedematous lesions and age greater than 60 years.46 Histopathology of tissue excised from these reactions reveals an intense immune reaction, often with multinucleated giant cells, with few or sparse acid‐fast bacilli visible.47 Mycobacteria are believed to be non‐viable, and thus mycobacterial cultures are often negative, but PCR and acid‐fast bacilli staining will usually remain positive.46
If clinically suspecting a paradoxical reaction, initial management involves excluding antibiotic failure from poor antibiotic compliance, as true treatment failure in adherent patients is rare.34 Antibiotic failure is usually clinically apparent, revealing large areas of yellow necrotic tissue with undermined wound edges, whereas in a paradoxical reaction there is pink granulating tissue intermingled with white connective tissue and no undermined wound edges.34 Further evidence can be gained on histopathological examination of a tissue biopsy specimen, which will show intense local inflammation in paradoxical reaction.47 If a paradoxical reaction is diagnosed, the antibiotic regimen should usually be continued at the same dose and duration. For severe and destructive paradoxical reactions, we recommend oral prednisolone 0.5–1.0 mg/kg daily tapered over four to eight weeks; antibiotics may be extended up to 12 weeks’ total duration in these cases, although experiments in mouse models suggest this may not be required.48 Fluctuant lesions may require aspiration or drainage, and severe reactions may require surgical debridement to preserve tissue and improve rates of healing as described above.42 Some clinicians favour pre‐emptive prednisolone, alongside antibiotic therapy, in patients with high risk oedematous lesions to prevent significant tissue loss.49
Wound care
Effective wound care is an essential component of Buruli ulcer management, particularly for deep and complex wounds. There are three stages of Buruli ulcer wound care:
- cleaning — active removal of necrotic and inflammatory tissue;
- healing — re‐epithelialisation once clean, and after resolution of inflammation; and
- protection — prevention of damage to the wound after healing and scar maturation.
Important principles to guide effective wound management and optimise progression through the three stages of wound care for Buruli ulcer are detailed in Box 4. Many patients will source their own wound care materials and manage their own dressing changes at home, but community and district nursing services can be arranged for patients who cannot manage this alone (eg, patients who are older, have a disability, or have sensory impairment) or require specialist wound care.50 Regular dressing assistance may also be available through primary health care providers and can significantly expedite wound healing, especially in clinics located in Buruli ulcer endemic areas with extensive experience. Many health care networks have a chronic wound service with multidisciplinary teams of experienced nurses, doctors and other health professionals that are available to assist if required, particularly for large or deep wounds that have significant exudate, require gauze or packing, or occur over a sensitive site.
Special considerations in population subgroups
Paediatric Buruli ulcer
Children aged 15 years or younger account for about 10% of reported cases. There is limited literature on paediatric Buruli ulcer, and the largest cohort study in the world included 52 children with a median age of eight years in Victoria.51 The site of lesion distribution was similar to adults, although a higher proportion were non‐ulcerative (oedematous or nodular lesions) and severe, and a lower proportion of children had multiple lesions. Children had a significantly shorter time to diagnosis and were less likely to experience adverse antibiotic effects, although they had higher rates of paradoxical reaction.
The recommended treatment of paediatric Buruli ulcer is oral rifampicin and clarithromycin for the same dose and duration as adults. Oral azithromycin 10 mg/kg per dose (maximum 500 mg) daily is an alternative agent currently used in select paediatric referral centres, and has the advantage of once daily dosing for patients where there is administration or tolerability issues with clarithromycin. Ciprofloxacin 15 mg/kg per dose (maximum 500 mg) orally twice a day is another possible alternative, although concerns remain with fluroquinolone administration in children, and poor palatability and tolerability.52 Some children may experience a brief period of gastrointestinal upset including anorexia, nausea and diarrhoea; this is usually self‐limiting and occurs within the first two weeks of treatment. Antibiotic side effects, including hepatic dysfunction and QTc prolongation, are rarely observed in children; and hepatic, renal or cardiac monitoring is usually not required in the absence of other comorbid conditions.
Managing children with Buruli ulcer can be challenging. Tolerance of prolonged antibiotic administration can be difficult, especially for children under six years of age who are usually unable to swallow tablets and therefore require large quantities of liquid antibiotic formulations. Disguising the taste and engaging child play therapy may be useful in supporting children to successfully complete the treatment. As large volumes are often required, treatment can be expensive because multiple bottles may need to be purchased every fortnight. This is compounded by the fact that rifampicin liquid formulations are not subsidised by the Pharmaceutical Benefits Scheme for M. ulcerans infection.
With regular dressings and antibiotic adherence, aggressive surgical intervention is usually not required. In addition, for children under six years of age, this cannot routinely be performed without sedation or anaesthesia. If dressing changes are poorly tolerated, we recommend consultation with a paediatric specialist. Paradoxical reactions have been reported in 39% of children.51 Early recognition of high risk wounds (eg, oedematous wounds) or clinical evidence of a paradoxical reaction after treatment initiation is important to minimise further tissue destruction. Children with severe paradoxical reaction (eg, rapid, destructive) should be commenced on oral prednisolone 0.5 mg/kg (maximum 40 mg) daily, which is carefully monitored and tapered over at least one month, due to the risk of paradoxical reaction recrudescence. Common adverse effects from high dose steroid therapy include increased appetite, weight gain, and emotional lability, although rarely lead to cessation of therapy.
Aboriginal and Torres Strait Islander people with Buruli ulcer
Australia has a publicly funded health care system that exists against a backdrop of significant inequity, limited access and underfunding of health care for Aboriginal and Torres Strait Islander people.53 Skin disease is highly prevalent in remote Aboriginal communities, and skin neglected tropical diseases, including scabies and strongyloidiasis, are endemic in northern Australia.54 Buruli ulcer may be uncommonly diagnosed in Aboriginal and Torres Strait Islander populations in Australia,19,55 but cases are associated with diagnostic delay, challenges with antibiotic adherence, and prolonged hospitalisation.56 For cases of Buruli ulcer that occur outside of major health care settings, it is important to consider any limitations to health care provision, specialist support, wound management, and ongoing care requirements. Buruli ulcers in non‐endemic regions are often larger, deeper and complex than those occurring in endemic regions, and may require modification of standard treatment protocols.
Prevention
Based on current understanding, the following measures are anticipated to reduce risk of Buruli ulcer:
- Enact mosquito bite avoidance strategies, with the use of mosquito repellents, protective clothing (ie, loose‐fitting clothes, covering exposed skin) and where possible, prevent mosquitos from entering the home (eg, installation of fly screens).57
- Minimise mosquito breeding opportunities by reducing pooled water (eg, pot plants).
- Wear gloves, long‐sleeved shirts and trousers when gardening or working outdoors.
- Protect cuts and abrasions with a wound dressing, and promptly wash any new scratches or cuts with soap in water, followed by a topical antiseptic and wound dressing.
- Minimise contact with Australian native possums and their excreta.
- There are no currently available vaccines for Buruli ulcer prevention. Mycobacterium bovis BCG vaccine may offer short term protection, but more research is needed before it can be recommended.58
Box 1 – Presentation (A), progression (B) and healing with scarring (C) of Buruli ulcer
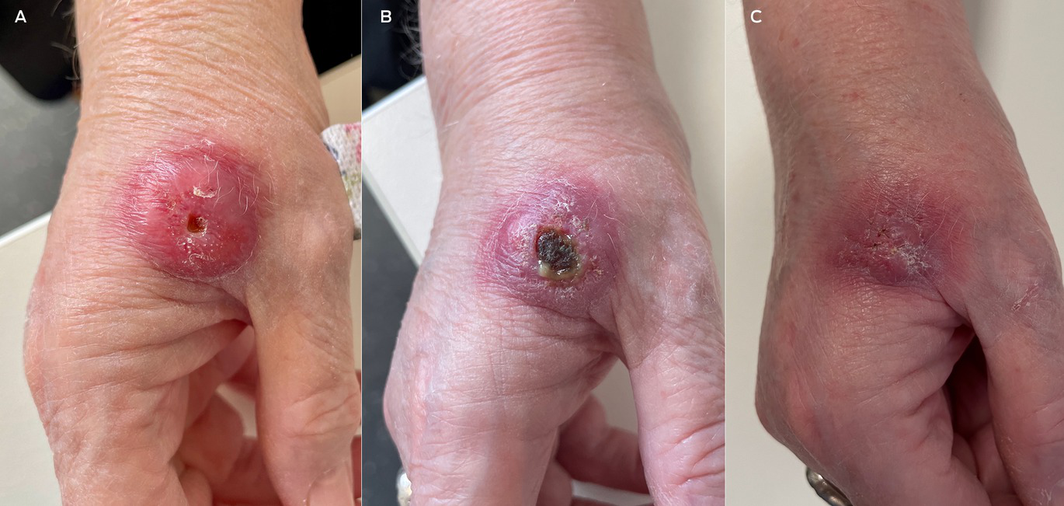
As awareness of Buruli ulcer improves, an increasing number of cases may present with early/pre‐ulcerative lesions (A); early recognition, diagnosis and treatment is likely to prevent advanced ulceration. Panel B shows progression, with typical undermined edges, surrounding induration and central necrosis. Lesions heal slowly and may leave an area of scarring (C). Patient consent: The patient gave written consent for the publication of these images.
Box 3 – Regions with confirmed Buruli ulcer transmission in Australia
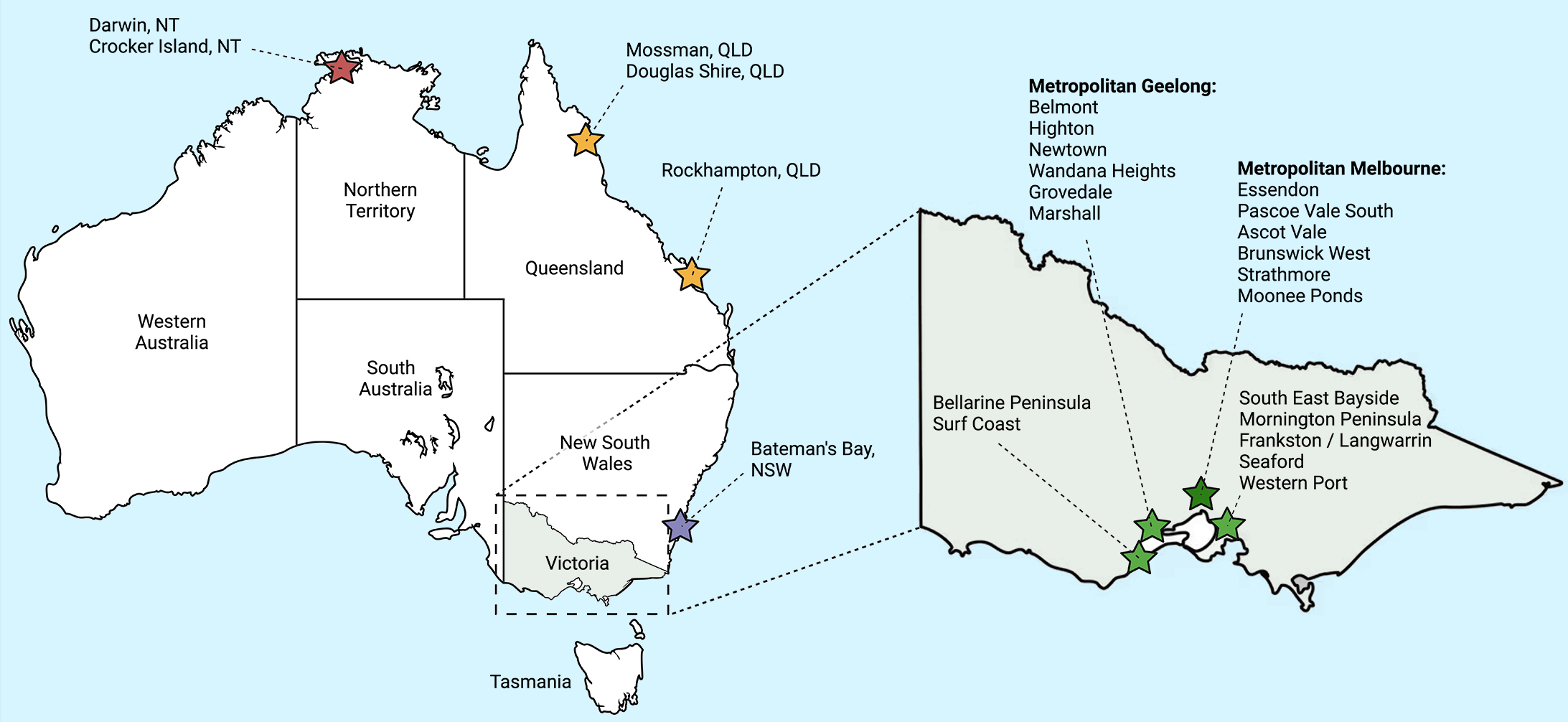
Figure created using BioRender.com.
Box 4 – Suggested measures to enable optimal wound care in people with Buruli ulcer
|
Measure |
Description |
||||||||||||||
|
|
|||||||||||||||
|
Removal of necrotic and inflammatory material is essential to enable wound healing and preventing paradoxical reactions and secondary infections. There are many ways to debride wounds effectively. Topical debriding agents can be purchased from community pharmacies without a prescription, although there is no strong evidence to recommend one particular agent over another and is often dependent upon clinician preference. Superficial debridement of necrotic tissue in the clinic setting may be performed by experienced clinicians. Surgical debridement is also effective. Buruli ulcer wounds can be washed with sterile water or saline, or during showering or bathing if the water is from a clean source. |
||||||||||||||
|
Open wounds should be covered at all times with an absorbent, breathable dressing. This prevents desiccation of healthy tissue, limits the risk of contamination and secondary infection of the wound, and can be effective in helping to debride wounds. Regular dressing changes should occur, usually every one to three days, depending on the exudate produced; earlier dressing change should occur if there is strikethrough. Dressings can also be used to protect wounds once healed. For fragile wounds, tight clothes and shoes over the ulcer should be avoided until wound healing has been established and matured, which may take months. Care should always be taken during dressing changes to avoid causing trauma to the wound. Podiatry and occupational therapy services may also be of benefit. |
||||||||||||||
|
The base of the wound should be kept moist, but heavily exudative wounds should be managed with absorbent dressings to prevent maceration, as this delays healing. Honey is a naturally occurring topical agent often used which provides moisture and nutrients for the wound and promotes wound healing through antibacterial properties. Other topical agents may also prevent moisture loss; there is no strong evidence to recommend one agent over another. |
||||||||||||||
|
Excess tissue swelling and limb oedema impairs wound healing; therefore, advise patients to elevate the affected limb and use appropriately fitted compression garments where required and tolerated. Consider prescribing diuretic agents for patients with superimposed peripheral oedema (eg, due to underlying congestive cardiac failure). |
||||||||||||||
|
Good nutrition is essential to promote wound healing. Take a detailed dietary history and consider involving a dietitian if poor nutrition is identified. Oral zinc and vitamin C supplementation may promote wound healing. |
||||||||||||||
|
Good blood supply is essential to enable healing. Assess peripheral pulses and venous drainage in patients with lower limb Buruli ulcers, but particularly in those at risk of arterial or venous insufficiency. If history or examination suggests poor vascular supply, refer for diagnostic imaging and specialist input. Encourage smoking cessation when possible. Encourage alcohol reduction with support from drug and alcohol services if required, as this may help adherence to wound care and help patients consciously protect the wound. Glycaemic control should be optimised as a priority for patients with diabetes and clinicians should have a low threshold to screen for it. Optimising the management of human immunodeficiency virus in patients with co‐infection improves outcomes, and consideration of drug interactions is important. |
||||||||||||||
|
If the Buruli ulcer involves a joint or affects limb movement, good positioning and regular movement is essential to prevent deformity and/or functional impairment. Temporary immobilisation of the affected area should only occur for the shortest time possible in cases that require surgical management (including skin graft) after seeking specialist advice from a surgeon and physiotherapist. Most importantly, early diagnosis and treatment of Buruli ulcer will ensure an optimal functional and cosmetic outcome. Specialist surgical opinion should always be sought in more severe cases that may result in permanent disability or disfigurement. |
||||||||||||||
|
To minimise scars, reduce transepidermal water loss using a regular emollient for all patients. In those at risk of hypertrophic or keloid scarring, consider the use of a silicone gel or sheet, as silicone hydrates scars and promotes alignment of collagen fibres. Referral to a specialist clinician, occupational therapist or physiotherapist should also be considered for these patients. Protect from sun exposure by applying a sunscreen with a sun protection factor of 50+ to the scar to reduce the risk of dyspigmentation and promote fading of early scar erythema. |
||||||||||||||
|
Ulcers that have significant necrotic material are classified as tetanus‐prone, so patients should be offered a tetanus booster vaccination, particularly if there are concerns about the adequacy of wound care. Although Buruli ulcer is usually painless, pain may develop during antibiotic treatment. However, if the clinical trajectory is atypical or pain is excessive, consider contiguous spread of the infection to deeper structures, secondary bacterial infection, or paradoxical reaction. There is currently no evidence for use of hyperbaric oxygen therapy to aid with wound healing. |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Stephen Muhi1,2
- Victoria RV Cox3
- Matthew O'Brien4
- Jonathan T Priestley5
- Jodie Hill5
- Adrian Murrie6
- Anthony McDonald7
- Peter Callan7
- Grant A Jenkin8
- N Deborah Friedman1,2
- Kasha P Singh1,2
- Callum Maggs7
- Peter Kelley9,10
- Eugene Athan7,11
- Paul DR Johnson1,12
- Daniel P O'Brien2,7
- 1 Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC
- 2 Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne, VIC
- 3 Menzies School of Health Research, Darwin, NT
- 4 Monash Children's Hospital, Melbourne, VIC
- 5 South Coast Medical, Blairgowrie, VIC
- 6 Sorrento Medical Centre, Sorrento, VIC
- 7 Barwon Health, Geelong, VIC
- 8 Monash Medical Centre, Melbourne, VIC
- 9 Peninsula Health, Melbourne, VIC
- 10 Eastern Health, Melbourne, VIC
- 11 Centre for Innovation in Infectious Disease and Immunology Research, Deakin University, Geelong, VIC
- 12 Austin Health, Melbourne, VIC
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
No relevant disclosures.
- 1. World Health Organization. Generic framework for control, elimination and eradication of neglected tropical diseases. Geneva: WHO, 2016. https://www.who.int/publications/i/item/WHO‐HTM‐NTD‐2016.6 (viewed Jan 2025).
- 2. World Health Organization. Buruli ulcer: the Global Health Observatory, 2024. Geneva: WHO, 2024. https://www.who.int/data/gho/data/themes/topics/buruli‐ulcer (viewed Jan 2025).
- 3. Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep 2018; 5: 247‐256.
- 4. Gunawardana G, Chatterjee D, George KM, et al. Characterization of novel macrolide toxins, mycolactones A and B, from a human pathogen, Mycobacterium ulcerans. J Am Chem Soc 1999; 121: 6092‐6093.
- 5. McNamara BJ, Blasdell KR, Yerramilli A, Smith IL, Clayton SL, Dunn M, et al. Comprehensive case–control study of protective and risk factors for Buruli ulcer, southeastern Australia. Emerg Infect Dis 2023; 29: 2032‐2043.
- 6. Blasdell KR, McNamara B, O'Brien DP, et al. Environmental risk factors associated with the presence of Mycobacterium ulcerans in Victoria, Australia. PLoS One 2022; 17: e0274627.
- 7. Xu RW, Stinear TP, Johnson PDR, O'Brien DP. Possum bites man: case of Buruli ulcer following possum bite. Med J Aust 2022; 216: 452‐453. https://www.mja.com.au/journal/2022/216/9/possum‐bites‐man‐case‐buruli‐ulcer‐following‐possum‐bite
- 8. Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis 2017; 11: e0005553.
- 9. Fyfe JA, Lavender CJ, Handasyde KA, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis 2010; 4: e791.
- 10. Trubiano JA, Lavender CJ, Fyfe JAM, et al. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Negl Trop Dis 2013; 7: e2463.
- 11. Yerramilli A, Tay EL, Stewardson AJ, et al. The location of Australian Buruli ulcer lesions — implications for unravelling disease transmission. PLoS Negl Trop Dis 2017; 11: e0005800.
- 12. Boyd SC, Athan E, Friedman ND, et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Aust 2012; 196: 341‐344. https://www.mja.com.au/journal/2012/196/5/epidemiology‐clinical‐features‐and‐diagnosis‐mycobacterium‐ulcerans‐australian
- 13. O'Brien DP, Friedman ND, McDonald A, et al. Clinical features and risk factors of oedematous Mycobacterium ulcerans lesions in an Australian population: beware cellulitis in an endemic area. PLoS Negl Trop Dis 2014; 8: e2612.
- 14. Steffen CM, Freeborn H. Mycobacterium ulcerans in the Daintree 2009–2015 and the mini‐epidemic of 2011. ANZ J Surg 2018; 88: E289‐E293.
- 15. Loftus MJ, Tay EL, Globan M, et al. Epidemiology of Buruli ulcer infections, Victoria, Australia, 2011–2016. Emerg Infect Dis 2018; 24: 1988‐1997.
- 16. Johnson PDR. Buruli ulcer in Australia. In: Pluschke G, Röltgen K; editors. Buruli ulcer: Mycobacterium ulcerans disease. Cham: Springer International Publishing, 2019; pp. 61‐76.
- 17. Department of Health and Human Services. Interactive infectious diseases surveillance reports [updated 6 Mar 2024; website]. Melbourne: Victorian Government, 2024. https://www.health.vic.gov.au/infectious‐diseases/local‐government‐areas‐surveillance‐report (viewed Jan 2025).
- 18. Office of the Chief Health Officer. Buruli ulcer clinician alert. Canberra: ACT Health, 2023. https://www.act.gov.au/__data/assets/pdf_file/0011/2398709/Buruli‐ulcer‐clinician‐alert‐Dec‐2023.pdf (viewed Jan 2025).
- 19. Nohrenberg M, Wright A, Krause V. Non‐tuberculous mycobacterial skin and soft tissue infections in the Northern Territory, Australia, 1989‐2021. Int J Infect Dis 2023; 135: 125‐131.
- 20. O'Brien DP, Jenkin G, Buntine J, et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust 2014; 200: 267‐270. https://www.mja.com.au/journal/2014/200/5/treatment‐and‐prevention‐mycobacterium‐ulcerans‐infection‐buruli‐ulcer‐australia
- 21. Sakyi SA, Aboagye SY, Darko Otchere I, Yeboah‐Manu D. Clinical and laboratory diagnosis of Buruli ulcer disease: a systematic review. Can J Infect Dis Med Microbiol 2016; 2016: 5310718.
- 22. O'Brien DP, Globan M, Fyfe JM, et al. Diagnosis of Mycobacterium ulcerans disease: be alert to the possibility of negative initial PCR results. Med J Aust 2019; 210: 416. https://www.mja.com.au/journal/2019/210/9/diagnosis‐mycobacterium‐ulcerans‐disease‐be‐alert‐possibility‐negative‐initial#:~:text=A%20negative%20PCR%20test%20result,ulcerative%20lesion%20is%20usually%20negative
- 23. Betts J, Tay EL, Johnson PD, et al. Buruli ulcer: a new case definition for Victoria. Commun Dis Intell (2018) 2020; https://doi.org/10.33321/cdi.2020.44.93.
- 24. Françoise Portaels, editor; World Health Organization. Laboratory diagnosis of Buruli ulcer: a manual for health care providers; 1st ed. Geneva: WHO, 2014. https://www.who.int/publications/i/item/9789241505703 (viewed Jan 2025).
- 25. Guarner J, Bartlett J, Whitney EAS, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis 2003; 9: 651‐656.
- 26. Phillips RO, Robert J, Abass KM, et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open‐label, non‐inferiority phase 3 trial. Lancet 2020; 395: 1259‐1267.
- 27. O'Brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south‐eastern Australian case series. Med J Aust 2007; 186: 58‐61. https://www.mja.com.au/journal/2007/186/2/outcomes‐mycobacterium‐ulcerans‐infection‐combined‐surgery‐and‐antibiotic
- 28. Friedman ND, Athan E, Walton AL, O'Brien DP. Increasing experience with primary oral medical therapy for Mycobacterium ulcerans disease in an Australian cohort. Antimicrob Agents Chemother 2016; 60: 2692‐2695.
- 29. Asiedu K, editor; World Health Organization. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers. Geneva: WHO, 2012. https://www.who.int/publications/i/item/9789241503402 (viewed Jan 2025).
- 30. Owusu E, Newman MJ, Addo KK, Addo P. In vitro susceptibility of Mycobacterium ulcerans isolates to selected antimicrobials. Can J Infect Dis Med Microbiol 2017; 2017: 5180984.
- 31. Ji B, Chauffour A, Robert J, et al. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 2007; 51: 3737‐3739.
- 32. O'Brien DP, Friedman ND, Cowan R, et al. Six vs eight weeks of antibiotics for small Mycobacterium ulcerans lesions in Australian patients. Clin Infect Dis 2020; 70: 1993‐1997.
- 33. Cowan R, Athan E, Friedman ND, et al. Mycobacterium ulcerans treatment — can antibiotic duration be reduced in selected patients? PLoS Negl Trop Dis 2015; 9: e0003503.
- 34. O'Brien DP, Friedman ND, Walton A, et al. Risk factors associated with antibiotic treatment failure of Buruli ulcer. Antimicrob Agents Chemother 2020; 64: e00722‐20.
- 35. Marsollier L, Honoré N, Legras P, et al. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob Agents Chemother 2003; 47: 1228‐1232.
- 36. Ashok A, Warner VM, Gardiner BJ. Multifocal cutaneous Mycobacterium ulcerans infection in a heart transplant recipient. Transpl Infect Dis 2024; 26: e14262.
- 37. O'Brien DP, Friedman ND, Cowan R, et al. Mycobacterium ulcerans in the elderly: more severe disease and suboptimal outcomes. PLoS Negl Trop Dis 2015; 9: e0004253.
- 38. O'Brien DP, Friedman D, Hughes A, et al. Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Intern Med J 2017; 47: 1011‐1019.
- 39. UpToDate. Interactions: levonorgestrel (IUD)/CYP3A4 inducers [website]. https://www.uptodate.com/drug‐interactions (viewed Oct 2024).
- 40. O'Brien DP, Friedman ND, McDonald A, et al. Wound healing: Natural history and risk factors for delay in Australian patients treated with antibiotics for Mycobacterium ulcerans disease. PLoS Negl Trop Dis 2018; 12: e0006357.
- 41. World Health Organization Collaborating Centre for Mycobacterium ulcerans. Clinician information [website]. Melbourne: Peter Doherty Institute for Infection and Immunity, 2024. https://www.doherty.edu.au/who‐cc‐m‐ulcerans/clinicians (viewed June 2024).
- 42. O'Brien DP, Callan P, Friedman ND, et al. Mycobacterium ulcerans disease management in Australian patients: the re‐emergence of surgery as an important treatment modality. ANZ J Surg 2019; 89: 653‐658.
- 43. Wadagni AC, Barogui YT, Johnson RC, et al. Delayed versus standard assessment for excision surgery in patients with Buruli ulcer in Benin: a randomised controlled trial. Lancet Infect Dis 2018; 18: 650‐656.
- 44. Tweedale B, Collier F, Waidyatillake NT, et al. Mycobacterium ulcerans culture results according to duration of prior antibiotic treatment: a cohort study. PLoS One 2023; 18: e0284201.
- 45. O'Brien DP, McDonald A, Callan P. Risk factors for recurrent Mycobacterium ulcerans disease after exclusive surgical treatment in an Australian cohort. Med J Aust 2014; 200: 86. https://www.mja.com.au/journal/2013/198/8/risk‐factors‐recurrent‐mycobacterium‐ulcerans‐disease‐after‐exclusive‐surgical#:~:text=Conclusions%3A%20Recurrence%20rates%20after%20exclusive,immunosuppression%20or%20positive%20histological%20margins
- 46. O'Brien DP, Robson M, Friedman ND, et al. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infect Dis 2013; 13: 416.
- 47. O'Brien DP, Robson ME, Callan PP, McDonald AH. “Paradoxical” immune‐mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med J Aust 2009; 191: 564‐566. https://www.mja.com.au/journal/2009/191/10/paradoxical‐immune‐mediated‐reactions‐mycobacterium‐ulcerans‐during‐antibiotic
- 48. Martins TG, Trigo G, Fraga AG, et al. Corticosteroid‐induced immunosuppression ultimately does not compromise the efficacy of antibiotherapy in murine Mycobacterium ulcerans infection. PLoS Negl Trop Dis 2012; 6: e1925.
- 49. O'Brien DP, Huffam S. Pre‐emptive steroids for a severe oedematous Buruli ulcer lesion: a case report. J Med Case Rep 2015; 9: 98.
- 50. Carville K. Wound care manual; 6th ed. Osborne Park (WA): Silver Chain Nursing Association, 2012.
- 51. Walker G, Friedman DN, O'Brien MP, et al. Paediatric Buruli ulcer in Australia. J Paediatr Child Health 2020; 56: 636‐641.
- 52. Patel K, Goldman JL. Safety concerns surrounding quinolone use in children. J Clin Pharmacol 2016; 56: 1060‐1075.
- 53. Zhao Y, Wakerman J, Zhang X, et al. Remoteness, models of primary care and inequity: Medicare under‐expenditure in the Northern Territory. Aust Health Rev 2022; 46: 302‐308.
- 54. Andrews RM, Kearns T, Connors C, et al. A regional initiative to reduce skin infections amongst Aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Negl Trop Dis 2009; 3: e554.
- 55. Steffen CM, Smith M, McBride WJH. Mycobacterium ulcerans infection in North Queensland: the “Daintree ulcer”. ANZ J Surg 2010; 80: 732‐736.
- 56. Mahony M, Hung TY, Cox V, et al. Complicated Mycobacterium ulcerans infection in a child in the Northern Territory. J Paediatr Child Health 2023; 59: 392‐394.
- 57. Quek TYJ, Athan E, Henry MJ, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis 2007; 13: 1661‐1666.
- 58. Muhi S, Stinear TP. Systematic review of M. bovis BCG and other candidate vaccines for Buruli ulcer prophylaxis. Vaccine 2021; 39: 7238‐7252.






Abstract
Introduction: Buruli ulcer, caused by Mycobacterium ulcerans, is increasing in incidence and spreading to new areas in southeast Australia. With increasing experience and emerging evidence, this consensus statement considers contemporary data to provide up‐to‐date recommendations to clinicians who may encounter this disease. The emergence of Buruli ulcer in previously non‐endemic areas highlights the importance of increasing clinician and community awareness of this disease.
Main recommendations and changes in management as a result of this consensus statement: