The known: Persistent symptoms can occur following COVID‐19 and may be associated with ongoing impairment.
The new: A survey of adults in Victoria, Australia, who had had a confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection showed that one in seven reported persistent new symptoms and being less than 80% recovered three months after the infection (meeting the survey criteria for clinical long COVID). One in five of those with clinical long COVID reported at least moderate impairment at 12 months after the infection.
The implications: Although more recent SARS‐CoV‐2 variants are less virulent, infections are likely to continue to cause persistent symptoms, and a minority of those affected will experience decreased function. Improved community understanding of long COVID is required, and health systems need to develop clear pathways for treating patients, especially for those with persistent impairment.
Although most patients return to their baseline state of health after having coronavirus disease 2019 (COVID‐19), some experience persistent symptoms. The most common of these are fatigue, exertional dyspnoea and cognitive difficulties.1 While some symptoms reflect the consequences of severe acute illness, often associated with pulmonary damage and prolonged hospitalisation, persistent symptoms predominantly occur in those not requiring hospitalisation, suggesting other pathological mechanisms.2
Research into the epidemiology of persistent symptoms after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been complicated by variations in definitions, sampling, vaccination rates, SARS‐CoV‐2 variants and health care contexts. International studies using the World Health Organization's definition of post‐COVID‐19 condition (long COVID)3 — symptoms at three months having been present for at least two months and not otherwise explained — have indicated that the incidence ranges from 7.5% to 41% for non‐hospitalised adults and is 38% for hospitalised adults.4 Pooled data from prospective studies of clinical populations indicate a 59% prevalence of at least one symptom at 12 months after the infection.5 Population‐based surveys have reported lower figures. The United Kingdom's Coronavirus Infection Survey, for example, followed 10 266 representative households from March 2021 and estimated a peak in November 2022, when 2.8% of the population reported unexplained symptoms occurring at least three months after an infection.6
The severity of persistent symptoms after COVID‐19 in Australia will likely differ from other countries. Public health restrictions kept the infection rate low early in the pandemic and most cases occurred after vaccination was widely available.7 The mortality from COVID‐19 in Australia, one measure of severity, has been less when compared with other parts of the world (0.2% in Australia compared with 0.9% elsewhere).8 The first Australian follow‐up study, which included 2000 general hospital patients, showed that 5% of patients reported persistent symptoms at three months after infection with SARS‐CoV‐2.6 A 2022 follow‐up of a pre‐COVID‐19 cohort reported that 13.9% of those who thought they had contracted COVID‐19 described ongoing symptoms lasting more than four weeks, and almost a quarter of them said that their day‐to‐day activities were affected “a lot”.9
To obtain further Australian data on persistent symptoms, functional impairment and health care utilisation, we conducted a survey of health outcomes after SARS‐CoV‐2 infection. We conducted the study within the Victorian Department of Health and sampled adults from the Victorian statewide database of confirmed infections (the Transmission and Response Epidemiology Victoria [TREVi] database). We aimed to quantify symptoms, functional impairment and service use following COVID‐19.
Methods
We conducted a stratified random health survey using a sample of adults (aged 18 years or over) from the TREVi database who had had a confirmed SARS‐CoV‐2 infection between 1 January 2020 and 31 October 2022. A stratified random sample of those confirmed to have had the infection was selected from the database (the COVID‐19‐positive group). Similarly, a stratified random sample of adults from TREVi who were considered close contacts, but self‐reported that they had not contracted COVID‐19, was selected (the control group). The samples were divided into periods reflecting stages of the pandemic in Victoria, including the predominant viral strains detected during those periods.
The study was designed in accordance with Strengthening the Reporting of Observational Studies in Epidemiology10 and Sex and Gender Equity in Research11 guidelines. The control group was included to account for health impacts on the general population occurring during the pandemic.12
The survey used was based on the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) WHO Clinical Characterisation Protocol,13 which included: measures of health status before and after the infection (on a 0–100 visual analogue scale where 100 means “the best health you can imagine” and 0 means “the worst health you can imagine”); past health diagnoses; severity of SARS‐CoV‐2 infection; medical complications; role or occupation; and function (based on the EQ‐5D‐5L version of the EuroQol instrument).14 Additional questionnaires were added to provide data on cognition (the Neuro‐QOL Cognitive Function short form),15 anxiety (the General Anxiety Disorder 7‐item scale)16 and depression (the 9‐item Patient Health Questionnaire),17 and questions on health service utilisation were included. The survey was available in English and 12 other languages. Participants in the control group were administered the same questionnaire, excluding the questions on acute COVID‐19. Pre‐survey health for the control group was determined using a reference date of 1 November 2021.
The primary outcome was clinical long COVID; this was based on the WHO case definition of post‐COVID‐19 condition (long COVID) plus — in keeping with the WHO Clinical Case Definition Working Group on Post‐COVID‐19 Condition (which recommends an impairment criterion)18 — a threshold of less than 80% recovery three months after infection. A further classification of long COVID with impairment was established; this was defined as moderate or greater problems with usual activities in the usual activities domain of the EQ‐5D‐5L.14
A power analysis was performed to determine a meaningful difference in the standardised measures of symptoms and function to a power of 90% at a 1% significance level. This analysis determined that this would be achieved with 12 000 participants in the COVID‐19‐positive group and 2000 participants in the control group. A nested survey of initial non‐responders was also conducted to explore the impact of responder bias and to aid an imputation analysis.
Statistical analysis
Continuous variables are reported as means with standard deviations, and the statistical significance of differences relating to these (between participants in the COVID‐19‐positive and control groups) was assessed using t or Wilcoxon rank‐sum tests, as appropriate. Categorical variables are reported as proportions and the statistical significance of differences relating to these was assessed using χ2 tests.
To limit the impact of non‐responders on the representativeness of estimates, selection weights were adjusted using propensity scores.19 These were calculated by means of a ridge regression model predicting the likelihood of a contacted sample responding to the survey, conditional on characteristics available for both respondents and non‐respondents.
The resultant weights were further adjusted to ensure estimates were representative of Victorians aged 18 years and over. These weights were adjusted according to age, gender, location and education.
A logistic regression analysis was performed to determine the risk factors for long COVID. The covariates entered into the model were sex, age group, timing of acute COVID‐19 (aligned to dominant SARS‐CoV‐2 strain), Index of Relative Socio‐economic Advantage and Disadvantage (by decile, based on residential postcode), household income, pre‐existing chronic condition, pre‐existing anxiety or depression, language spoken at home, severity of acute COVID‐19 infection, highest level of education, Medicare access, private health insurance, and Primary Health Network catchment area (based on residential postcode).
Ethics approval
The health survey was approved by the Royal Melbourne Hospital Human Research Ethics Committee (HREC/78955/MH‐2021‐282352).
Results
Stratified samples of 118 851 people who had had COVID‐19 and 34 481 close contacts were extracted from a total of 2 020 645 people who had had COVID‐19 and a total of 174 305 close contacts. The data collection resulted in 12 688 survey completions. Of those who had been COVID‐19‐positive, 21 258 people responded (17.9%), of whom 11 174 substantially completed the questionnaire (9.4% of the sample). Of the close contacts, 3045 people responded (8.8%), of whom 1514 confirmed their COVID‐19‐negative status and substantially completed the survey (4.4% of the sample) (Supporting Information, tables 1 and 2, and figures 1 and 2).
The baseline characteristics of the COVID‐19‐positive group and control group respondents are detailed in Box 1. The stratified sample closely matched 2021 Victorian census data for age.20 The proportion of respondents in the 18–29‐year age group was higher than in the general population and the proportion of respondents in the 30–49‐year group was lower than in the general population (Supporting Information, table 2). The weighting process also accounted for non‐responders, who were targeted for a brief, follow‐up survey to understand their characteristics and health outcomes (Supporting Information, table 3).
The weighted survey data are used for figures presented throughout the remaining sections of this article. Weighted estimates can differ significantly from raw estimates, as they are adjusted for actual population distribution. As such, the numerator and proportion presented in the text may not concur with the respondent denominator in each instance.
The mean time between infection and survey completion was 12.6 months (SD, 0.46 months). The mean health status before infection was 85.1 (SD, 0.16), as compared with 80.1 (SD, 0.48) before survey completion for the control group (P < 0.001)
Outcomes
At the time of the survey, among the 11 174 respondents who had had COVID‐19:
- 4560 respondents (39.1%; 95% CI, 37.9–40.3%) reported at least one non‐pre‐existing symptom that had been present for least two months at three months after infection, which was classified as WHO‐defined long COVID;
- 1656 respondents (14.2%; 95% CI, 13.4–15.0%) reported at least one persistent new symptom and less than 80% recovery at three months, which was classified as clinical long COVID; and
- 535 respondents (3.2%; 95% CI, 2.6–3.8%) met the survey criteria for long COVID and reported at least moderate problems with usual activities (Supporting Information, table 4), which was classified as long COVID with persistent impairment.
This meant that 22.6% (95% CI, 20.0–25.2%) of respondents classified as having clinical long COVID described at least moderate problems with their usual activities.
Based on these results, an estimated 756 000 (95% CI, 714 000–799 000) adult Victorians may have been experiencing clinical long COVID at the time of the survey. This includes an estimated 173 000 (95% CI, 149 000–196 000) adult Victorians experiencing symptoms substantially affecting their daily activities one year after infection. In comparison to the COVID‐19‐positive respondents, 216 of those in the control group (20.8%; 95% CI, 18.5–23.1%) reported at least one persistent new symptom emerging after the reference date.
Symptoms
The most common symptoms reported were fatigue (feeling tired or fatigued, feeling physically weak, unable to exercise at usual level), impared neurocognition (brain fog, word finding), exertional dyspnoea and impaired sleeping. All symptoms surveyed occurred more frequently in the COVID‐19‐positive group as compared with controls (Box 2 and Supporting Information, table 5).
Return to work
The number of COVID‐19‐positive respondents who were employed at the time they contracted COVID‐19 was 8064 (70.4%; 95% CI, 69.2–71.6%), while 7826 (68.3%; 95% CI, 67.1–69.5%) were employed at the time of the survey — a decrease of 2.1 percentage points, although the confidence intervals overlap. For the control group, there was no statistically significant difference between the number employed in November 2021 (689, 67.7%; 95% CI, 65.0–70.4%) and at the time of the survey (675, 66.3%; 95% CI, 63.6–69.1%).
Risk factors
The multivariate analysis was completed using data from 3076 respondents with WHO‐defined long COVID. The covariates associated with an increased risk of long COVID were female sex, age 40–49 years (as compared with 18–29 years), requiring medical assistance for acute COVID‐19 (especially hospital admission), chronic condition before the infection, and having problems with anxiety or depression before the infection (Box 3). Infection dates when the Omicron or Delta strains were dominant, asymptomatic COVID‐19, higher household income, and being aged 70–79 years were associated with a decreased risk of having clinical long COVID (Box 3).
Health care utilisation
Close to one in four COVID‐19‐positive respondents (2734, 23.5%; 95% CI, 22.4–24.5%) said they had received some health assistance for persistent symptoms following COVID‐19. Of the COVID‐19‐positive respondents who had received assistance, 2033 (75.6%; 95% CI, 71.8–79.4%) had seen a general practitioner or family doctor, 270 (10.0%; 95% CI, 8.6–11.5%) reported receiving specialist care and 164 (6.1%; 95% CI, 5.0–7.2%) reported receiving hospital assistance (including in‐home care). For respondents with clinical long COVID, 1021 (61.6%; 95% CI, 58.6–64.7%) reported receiving assistance for persistent symptoms, 889 of whom (87.1%; 95% CI, 80.2–94.0%) received this from a general practitioner or family doctor.
Discussion
In this survey of an adult Australian state population, almost 40% of respondents who had had COVID‐19 had at least one new symptom one year after infection, which was twice as many as for control group respondents over a similar period. One in seven reported less than 80% recovery at three months, and 3% had at least moderate functional impairment at one year. A quarter of respondents with persistent symptoms received specific health care, mostly in primary care. The proportion of respondents with long COVID halved over the survey period, corresponding with the emergence of less virulent strains and higher rates of vaccination.
A strength of this study is its use of a statewide database that collected data on people with a confirmed case of COVID‐19 during a time of mandatory reporting. This method is unlikely to be replicated given changing patterns of testing and reporting. The use of a control group allowed for confidence that the symptoms described could not be solely attributed to more general impacts of the pandemic. The absence of serological confirmation for this group, however, means that some reported symptoms may have been due to undiagnosed COVID‐19. Also, because data were collected via a survey, new symptoms due to an unrelated medical condition may have been attributed to COVID‐19, although this is unlikely to have had a major impact on our findings as the symptoms reported are typical of long COVID and not common in new unrelated conditions. Despite a rigorous multistep contact strategy, the response rate was modest, although consistent with that of similar surveys. The impact of responder bias cannot be fully elucidated, although the non‐responder survey results suggested only a modest bias towards responders being more symptomatic. Consequently, we cannot extrapolate prevalence from our data with confidence. That said, the percentage of people who reported at least one symptom at three months (39.1%) is close to that which has been reported for a similar random community‐based sample in England (37.7%).21 However, we can be more confident about the measures of change over time and results of the logistic regression analysis, which are less likely to be affected by the response rate.
Of the risk factors for long COVID that we identified, female sex and past medical illness align with previous evidence.22 While early studies indicated older age as a risk factor, more recent studies have also identified the 40–49‐year age group as highly vulnerable,22 as was the case in our study. This age group corresponds with an age of relative immune robustness, in keeping with evidence of immune dysregulation in patients with long COVID.23 The idea that people with pre‐existing anxiety and depression are at greater risk of developing long COVID has been noted in one previous study,24 but this association does not in itself support the notion that long COVID is a psychological condition. Most cases of long COVID occurred in those without a self‐reported history of mental health problems.
The notion that persistent symptoms occur following COVID‐19 is well established. According to our data, the burden of persistent symptoms may be less common with less virulent strains of the virus and in people who have been vaccinated.25 Future burden will be governed by rates of infection. What has been less well described previously is the impact of persistently impaired function. The report of a similar percentage of people with at least moderate impairment in function at 12 months measured using the EQ‐5D‐5L in a cohort from South Korea26 adds weight to this occurring in about 3% of those contracting COVID‐19.
A report from the House of Representatives Standing Committee on Health, Aged Care and Sport has recommended primary care‐based multidisciplinary treatment, led by general practitioners, as the model of care for managing long COVID.27 Our results suggest that two‐thirds of those with persistent symptoms are already engaging with their general practitioners, which supports this approach. This model is well suited to the large majority of people who recover over time. For those with persistent impairment, however, current access to allied health over the calendar year appears insufficient. Meanwhile many long COVID‐specific rehabilitation programs established during the pandemic have now closed. The optimal model of care for patients with persistent impairment, including the role of specialist multidisciplinary rehabilitation services and expanded access to allied health, remains an open question and should be a focus of future research.
The majority of respondents in our study who had had COVID‐19 were working age adults. People returning to work at a reduced capacity,28 and the economic impact,29 has been described previously. It is notable that employment entitlements around sick leave, especially in younger people, are not well aligned to the recovery needs of those with long COVID. The financial impact of a reduced capacity to engage in previous roles adds to the psychological burden arising from physical symptoms, functional impairment and social disengagement, all of which predispose people to secondary mental health conditions.30 Increased awareness and education about persistent symptoms after COVID‐19 will enhance the necessary collaboration between patients, health practitioners and employers that is required to formulate individual return‐to‐work plans. Targeted programs designed to support preservation of roles and graded partial return to work, analogous to the JobKeeper support measure, may enhance recovery in the minority of people with persistent impairment. The efficacy and cost‐effectiveness of such an approach represents a further avenue for investigation.
In conclusion, persistent symptoms are common after a COVID‐19 infection. While most patients with long COVID can be treated in primary care, pathways for the optimal management of those with persistent impairment need to be further developed.
Box 1 – Characteristics of the 12 688 health survey respondents — unweighted
|
|
Number (%) of respondents |
|
|||||||||||||
|
Characteristic |
COVID‐19‐positive group (n = 11 174) |
Control group (n = 1514) |
P |
||||||||||||
|
|
|||||||||||||||
|
Gender |
|
|
|
||||||||||||
|
Man |
4211 (37.7%) |
654 (43.2%) |
< 0.000 |
||||||||||||
|
Woman |
6872 (61.5%) |
842 (55.6%) |
< 0.000 |
||||||||||||
|
Non‐binary |
66 (0.6%) |
12 (0.8%) |
0.442 |
||||||||||||
|
Different term |
9 (0.1%) |
2 (0.1%) |
0.862 |
||||||||||||
|
Prefer not to say |
16 (0.1%) |
4 (0.3%) |
0.442 |
||||||||||||
|
Age |
|
|
|
||||||||||||
|
18–29 years |
2349 (21.2%) |
270 (18.1%) |
0.006 |
||||||||||||
|
30–39 years |
2028 (18.3%) |
239 (16.0%) |
0.033 |
||||||||||||
|
40–49 years |
1936 (17.5%) |
226 (15.1%) |
0.027 |
||||||||||||
|
50–59 years |
1782 (16.1%) |
283 (18.9%) |
0.006 |
||||||||||||
|
60–69 years |
1347 (12.1%) |
229 (15.3%) |
0.001 |
||||||||||||
|
≥ 70 years |
1648 (14.9%) |
248 (16.6%) |
0.086 |
||||||||||||
|
Highest level of education |
|
|
|
||||||||||||
|
Primary school |
1084 (9.9%) |
185 (12.5%) |
0.003 |
||||||||||||
|
High school |
1530 (14.0%) |
206 (13.9%) |
0.920 |
||||||||||||
|
Diploma |
3104 (28.4%) |
464 (31.3%) |
0.026 |
||||||||||||
|
Bachelor degree |
2828 (25.9%) |
333 (22.4%) |
0.004 |
||||||||||||
|
Postgraduate degree |
2370 (21.7%) |
296 (19.9%) |
0.129 |
||||||||||||
|
Chronic condition |
|
|
|
||||||||||||
|
Yes |
1266 (10.5%) |
192 (11.4%) |
0.303 |
||||||||||||
|
Private health insurance |
|
|
|
||||||||||||
|
Yes |
6693 (59.9%) |
880 (58.1%) |
0.196 |
||||||||||||
|
Income |
|
|
|
||||||||||||
|
< $10 000 |
394 (3.9%) |
50 (3.7%) |
0.742 |
||||||||||||
|
$10 000 to < $20 000 |
466 (4.7%) |
80 (5.9%) |
0.046 |
||||||||||||
|
$20 000 to < $30 000 |
573 (5.7%) |
79 (5.9%) |
0.890 |
||||||||||||
|
$30 000 to < $40 000 |
638 (6.4%) |
106 (7.9%) |
0.044 |
||||||||||||
|
$40 000 to < $50 000 |
666 (6.7%) |
103 (7.6%) |
0.195 |
||||||||||||
|
$50 000 to < $60 000 |
797 (8.0%) |
119 (8.8%) |
0.298 |
||||||||||||
|
$60 000 to < $80 000 |
1258 (12.6%) |
185 (13.7%) |
0.250 |
||||||||||||
|
$80 000 to < $100 000 |
1137 (11.4%) |
173 (12.8%) |
0.122 |
||||||||||||
|
$100 000 to < $125 000 |
1073 (10.7%) |
147 (10.9%) |
0.874 |
||||||||||||
|
$125 000 to < $150 000 |
880 (8.8%) |
83 (6.2%) |
0.001 |
||||||||||||
|
$150 000 to < $200 000 |
947 (9.5%) |
100 (7.4%) |
0.017 |
||||||||||||
|
≥ $200 000 |
1172 (11.7%) |
122 (9.1%) |
0.005 |
||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019. |
|||||||||||||||
Box 2 – Proportions of survey respondents who reported new and persistent symptoms (two months or over) at the time of the survey — weighted
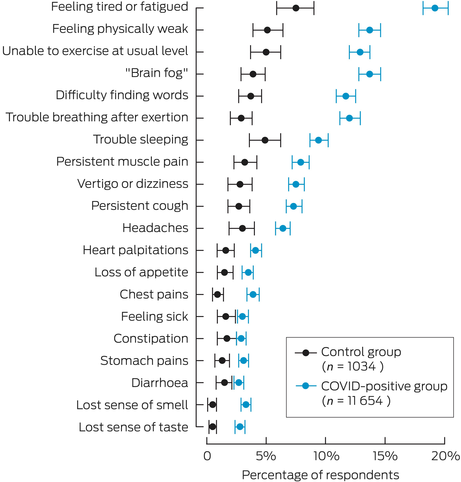
COVID‐19 = coronavirus disease 2019.
Box 3 – Risk factors for being classified as having clinical long COVID, based on data for 3076 people who had been COVID‐19‐positive between 1 January 2020 and 31 October 2022
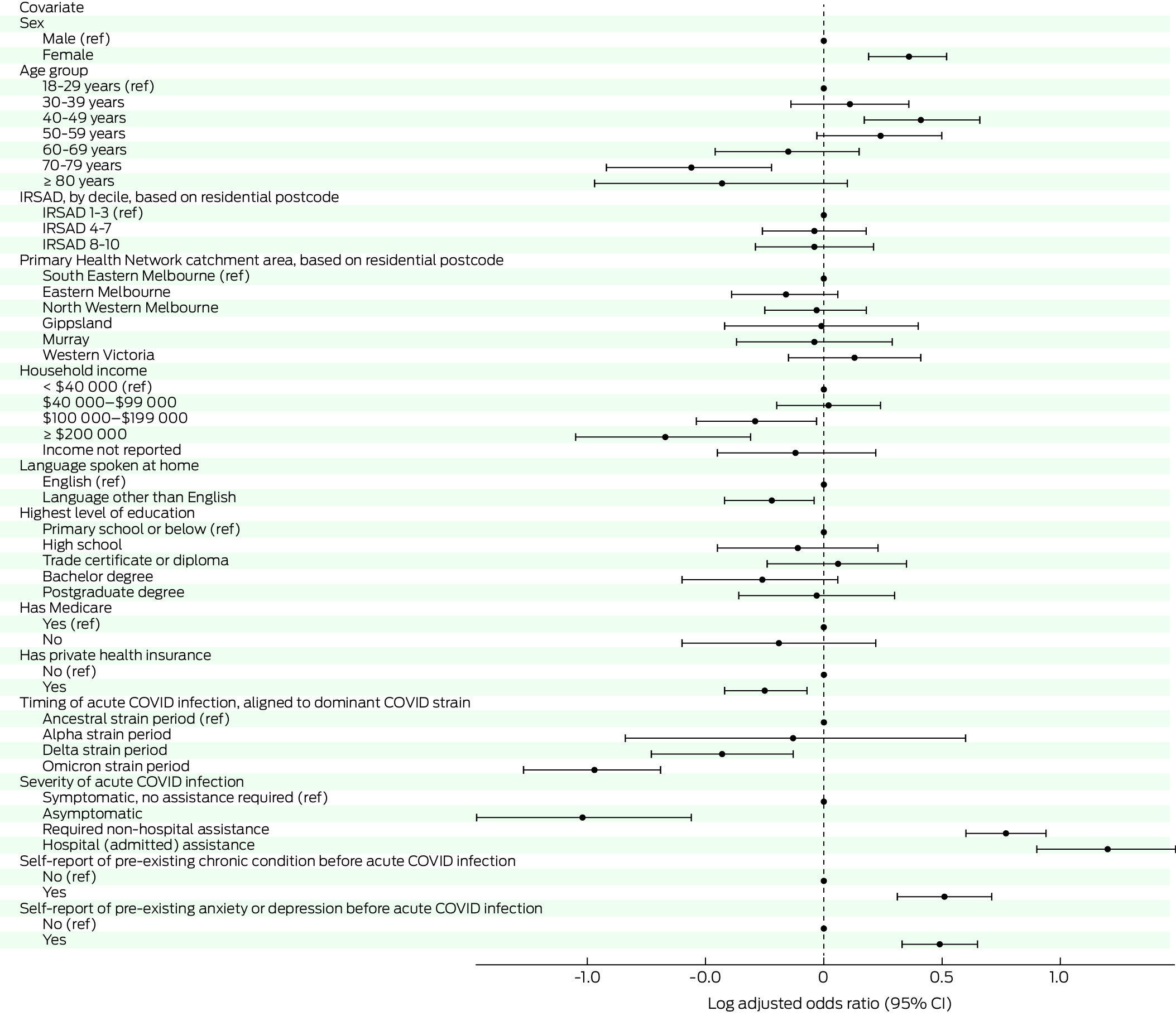
COVID‐19 = coronavirus disease 2019; IRSAD = Index of Relative Socio‐economic Advantage and Disadvantage; ref = reference category.
Received 12 February 2024, accepted 21 August 2024
- Alex Holmes1,2
- Lance Emerson3
- Louis B Irving1
- Emma Tippett4
- Jeffrey M Pullin3
- Julie Young3
- David A Watters5,6
- Adina Hamilton3
- 1 Royal Melbourne Hospital, Melbourne, VIC
- 2 University of Melbourne, Melbourne, VIC
- 3 Department of Health, Melbourne, VIC
- 4 Clinic Nineteen, Melbourne, VIC
- 5 Deakin University, Geelong, VIC
- 6 Safer Care Victoria, Melbourne, VIC
Open access:
Open access publishing facilitated by the University of Melbourne, as part of the Wiley – the University of Melbourne agreement via the Council of Australian University Librarians.
Data Sharing:
Survey data can be made available on application to the Victorian Department of Health, pending fulfillment of the relevant requirements. Please submit requests at https://vahi.vic.gov.au/ourwork/data‐linkage/apply.
We acknowledge the funding provided by the Victorian Department of Health. This funding was used to implement the survey using an external data collection provider; this included project design and development, survey implementation, preliminary analysis and weighting, and preliminary reporting. Substantial further analyses and reporting were completed by departmental analysts. We also acknowledge the contribution of: members of the Victorian Post‐Acute COVID‐19 Study group; members of the Victorian Department of Health's Post‐Acute COVID‐19 Data Steering Committee; members of the Victorian Long COVID Conference organising committee; and Zahid Ansari, Alison Markwick, Daisy Doan and Kate Riley Sandler from the Victorian Department of Health.
The study was conducted within and funded by the Victorian Department of Health. Lance Emerson, Jeffrey Pullin, Julie Young, and Adina Hamilton contributed to this manuscript through the course of their employment at the Victorian Department of Health. David Watters was, at the time of this study, seconded from Barwon Health to Safer Care Victoria. Alex Holmes, Louis Irving and Emma Tippett did not receive any payment from the Victorian Department of Health.
- 1. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626‐631.
- 2. Castanares‐Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022; 54: 1473‐1487.
- 3. World Health Organization. Post COVID‐19 condition (long COVID). 7 Dec 2022. https://www.who.int/europe/news‐room/fact‐sheets/item/post‐COVID‐19‐condition (viewed Oct 2023).
- 4. Nittas V, Gao M, West EA, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev 2022; 43: 1604501.
- 5. Mudgal SK, Gaur R, Rulaniya S, et al. Pooled prevalence of long COVID‐19 symptoms at 12 months and above follow‐up period: a systematic review and meta‐analysis. Cureus 2023; 15: e36325.
- 6. Office for National Statistics (UK). Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK: 2 February 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2february2023 (viewed Aug 2023).
- 7. Australian Institute of Health and Welfare. Long COVID in Australia – a review of the literature (AIHW Cat. No. PHE 318). Canberra: AIHW, 2022. https://www.aihw.gov.au/reports/covid‐19/long‐covid‐in‐australia‐a‐review‐of‐the‐literature/summary (viewed Oct 2023).
- 8. World Health Organization. WHO COVID‐19 dashboard. https://covid19.who.int (viewed Oct 2023).
- 9. Biddle N, Korda R. The experience of COVID‐19 in Australia, including long‐COVID – evidence from the COVID‐19 Impact Monitoring Survey series. Canberra: Australian National University, 2022. https://csrm.cass.anu.edu.au/research/publications/experience‐covid‐19‐australia‐including‐long‐covid‐evidence‐covid‐19‐impact (viewed Oct 2023).
- 10. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806‐808.
- 11. Heidari S, Babor TF, De Castro P, et al. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 2016; 1: 2.
- 12. Amin‐Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 2021; 27: 1129‐1130.
- 13. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985.
- 14. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011; 20: 1727‐1736.
- 15. Cella D, Lai JS, Nowinski CJ, et al. Neuro‐QOL: brief measures of health‐related quality of life for clinical research in neurology. Neurology 2012; 78: 1860‐1867.
- 16. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med 2006; 166: 1092‐1097.
- 17. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606‐613.
- 18. Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post‐COVID‐19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22: e102‐e7.
- 19. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41‐55.
- 20. Australian Bureau of Statistics. Snapshot of Victoria. Canberra: ABS, 2022. https://www.abs.gov.au/articles/snapshot‐vic‐2021 (viewed Oct 2023).
- 21. Whitaker M, Elliott J, Chadeau‐Hyam M, et al. Persistent COVID‐19 symptoms in a community study of 606,434 people in England. Nat Commun 2022; 13: 1957.
- 22. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non‐hospitalized adults. Nat Med 2022; 28: 1706‐1714.
- 23. Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild‐to‐moderate SARS‐CoV‐2 infection. Nat Immunol 2022; 23: 210‐216.
- 24. Wang S, Quan L, Chavarro JE, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness before infection with risk of post‐COVID‐19 conditions. JAMA Psychiatry 2022; 79: 1081‐1091.
- 25. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long COVID symptoms after COVID‐19 vaccination: community based cohort study. BMJ 2022; 377: e069676.
- 26. Kim Y, Bae S, Chang HH, Kim SW. Long COVID prevalence and impact on quality of life 2 years after acute COVID‐19 [author correction]. Sci Rep 2023; 13: 11960.
- 27. House of Representatives Standing Committee on Health, Aged Care and Sport. Sick and tired: casting a long shadow. Inquiry into long COVID and repeated COVID infections. Canberra: Commonwealth of Australia, 2023. https://www.aph.gov.au/Parliamentary_Business/Committees/House/Health_Aged_Care_and_Sport/LongandrepeatedCOVID/Report (viewed Oct 2023).
- 28. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019.
- 29. Cutler DM. The costs of long COVID. JAMA Health Forum 2022; 3: e221809.
- 30. Naidu SB, Shah AJ, Saigal A, et al. The high mental health burden of “Long COVID” and its association with on‐going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J 2021; 57: 2004364.






Abstract
Objective: To determine the impact of persistent symptoms after coronavirus disease 2019 (COVID‐19) in an Australian population.
Design, setting, participants: We conducted a statewide health survey of a stratified random sample of adults who had had a confirmed acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection (COVID‐19‐positive group) and their close contacts (control group). The sample was drawn from Victoria's COVID‐19 database between January 2020 and October 2022. Data were collected from 12 688 survey respondents between September 2022 and April 2023 (11 174 in the COVID‐19‐positive group and 1514 in the control group).
Main outcome measures: Persistent new symptoms, recovery, and daily function using validated questionnaires for fatigue, neurocognitive symptoms, anxiety, depression and quality of life.
Results: At a mean of 12.6 months after infection, 4560 respondents in the COVID‐19‐positive group (39.1%; 95% CI, 37.9–40.3%) reported at least one persistent new symptom, compared with 216 respondents in the control group (20.8%; 95% CI, 18.5–23.1%). A total of 1656 respondents (14.2%; 95% CI, 13.4–15.0%) were classified as having clinical long COVID using the criteria of at least one persistent new symptom and less than 80% recovery three months after the infection. Of the respondents with clinical long COVID, 535 (3.2%; 95% CI, 2.6–3.8%) reported at least moderate problems with usual activities at 12 months after their infection. The proportion of respondents with clinical long COVID was lower for those with more recent infections. The risk factors for clinical long COVID were female sex, age 40–49 years, infection severity, chronic illness, and past anxiety or depression. Factors associated with a decreased risk of having clinical long COVID included infection when the Omicron strain was dominant and infection when the Delta strain was dominant, as compared with when the ancestral strain of the virus was dominant.
Conclusion: Persistent symptoms after COVID‐19 are common, though with a lower incidence following infection from less virulent strains. Although long COVID can be largely managed in primary care, a minority of people who have persistent symptoms and impaired function may require specialist care pathways, the effectiveness of which should be a focus of future research.