Reactive infectious mucocutaneous eruption (RIME) is a severe mucocutaneous reaction most frequently seen in children and adolescents after an infectious respiratory illness, particularly Mycoplasma pneumoniae infections.1 It is characterised by prominent oral mucositis, conjunctivitis, and urethral involvement. Cutaneous manifestations are usually limited; the most frequent are vesicobullous eruptions.2 RIME is distinguished from Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) because it typically has an infectious cause, a milder course, and a favourable prognosis.1
In this article, we review the cases of thirteen children with RIME diagnosed by a specialist paediatric dermatologist and managed at the Sydney Children's Hospital at Randwick, a New South Wales referral centre, during 1 March – 31 May 2024. We extracted data and photographs from their medical records with the informed consent of their parents or guardians. The study was approved by the Sydney Children's Hospitals Network Human Research Ethics Committee (2024/ETH01030).
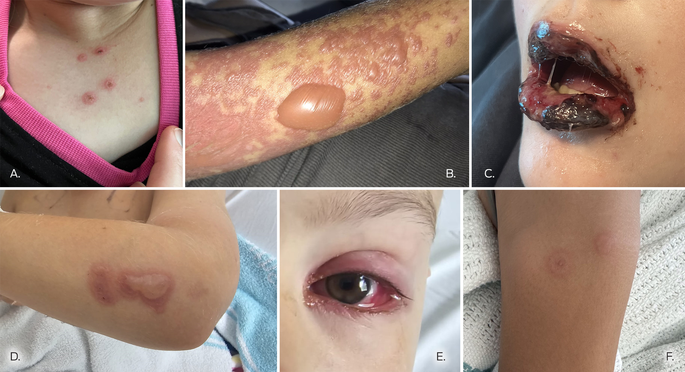
The mean age of the thirteen children (eight boys, five girls) was 11.7 years (range: 7–16 years); twelve were polymerase chain reaction (PCR)‐positive for M. pneumoniae. The mean length of respiratory illness preceding the mucocutaneous eruption was 7.4 days (range, 5–10 days). Oral manifestations were evident in all thirteen children, including haemorrhagic crusted lips, mucositis, and lip swelling. Seven children had cutaneous symptoms, limited in six to small targetoid lesions or bullae; eight children had ocular conjunctivitis, and four had urethral lesions (Box; Supporting Information).
All thirteen children received standard supportive care, including sodium bicarbonate mouthwash, lignocaine mouth rinse, paracetamol, ocular lubricants, soft white paraffin for their lips, a nasogastric tube for fluid and nutrition management, and pain team support. Six children received systemic immunosuppressants, five oral corticosteroids, and one intravenous immunoglobulin. The mean duration of hospitalisation was 7.4 (standard deviation [SD], 2.3) days; the mean length did not differ significantly between those who received immunosuppressants (8.2 [SD, 1.0] days) and those who did not (6.7 [SD, 0.8] days). The conditions of all thirteen children had improved within 14 days of admission, and they were discharged home (Supporting Information).
Outbreaks of M. pneumoniae‐associated mucocutaneous eruptions have been reported previously.3 Other infectious causes that can trigger the condition include Chlamydia pneumoniae and common respiratory viruses;1 the pathogenesis of the eruptions is unknown. The clinical presentation of RIME is similar to that of SJS/TEN, primarily a drug‐induced phenomenon with significant chronic sequelae and mortality of about 15%.4 However, RIME is a post‐infection condition; no recent fatal cases and few chronic sequelae have been reported.2 The diagnostic criteria for RIME include a mucocutaneous eruption in a child that affects less than 10% of body surface area, a recent respiratory illness and evidence of the infectious trigger, and no evidence for a medication‐related cause.2,5 All cases should be reviewed by a dermatologist as early as possible, and the diagnosis reconsidered should extensive epidermal detachment or a suspicious drug history be determined. Herpes simplex virus gingivostomatitis should be considered in the differential diagnosis.
Supportive care is the mainstay of treatment, together with multidisciplinary care by paediatricians, pain specialists, ophthalmologists, and dermatologists. Saline‐soaked gauze and soft paraffin to the lips prevent excessive crusting and fissuring, and reduce pain. Topical anaesthetic (oral lignocaine solution) reduces pain and improves oral intake; paraffin and topical anaesthesia for urethral lesions improves voiding. If urinary retention or constipation caused by voiding pain develops, consultation with a urologist and catheterisation should be considered. Ocular lubrication and ophthalmology reviews are important for averting complications such as synechiae and conjunctival ulceration. Although antibiotics may be required for the underlying respiratory illness, they do not relieve the symptoms of RIME or alter its course. It is unclear whether immunosuppressant treatment leads to better outcomes; they did not reduce hospital length of stay in our case series. We consider systemic treatment when the differential diagnosis between SJS/TEN and RIME is uncertain, or progressive new cutaneous lesions develop after five to seven days of conservative treatment.
RIME can follow infections with M. pneumoniae or other respiratory pathogens in children and adolescents. It causes significant pain and distress for those affected and their families, but the prognosis is good, with recovery within 14 days expected. Supportive multidisciplinary treatment ensures the best outcomes.
Received 8 June 2024, accepted 14 August 2024
- 1. Ramien ML. Reactive infectious mucocutaneous eruption: Mycoplasma pneumoniae‐induced rash and mucositis and other parainfectious eruptions. Clin Exp Dermatol 2021; 46: 420‐429.
- 2. Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae‐induced rash and mucositis as a syndrome distinct from Stevens–Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015; 72: 239‐45.
- 3. Olson D, Watkins LKF, Demirjian A, et al. Outbreak of Mycoplasma pneumoniae‐associated Stevens–Johnson syndrome. Pediatrics 2015; 136: e386‐e394.
- 4. Wasuwanich P, So JM, Chakrala TS, et al. Epidemiology of Stevens–Johnson syndrome and toxic epidermal necrolysis in the United States and factors predictive of outcome. JAAD Int 2023; 13: 17‐25.
- 5. Pan CX, Hussain SH. Recurrent reactive infectious mucocutaneous eruption: a retrospective cohort study. J Am Acad Dermatol 2023; 89: 361‐364.






Open access:
Open access publishing facilitated by the University of Sydney, as part of the Wiley – the University of Sydney agreement via the Council of Australian University Librarians.
Data Sharing:
The data underlying this study are included in the Supporting Information file; electronic medical records are not available for sharing.
No relevant disclosures.