Iron deficiency is the most common micronutrient deficiency worldwide1 and the predominant cause of anaemia, which affects one‐quarter of the global population.2
In Australia, 22.3% of women have depleted iron stores (serum ferritin level < 30 μg/L), with pre‐menopausal women disproportionately affected.3 In contrast, 3.5% of men are iron deficient.3
The Australian Red Cross Lifeblood implemented routine ferritin level testing in August 2023 for new whole blood donors (105 069 in 2023),4 with expanded testing to include returning blood donors in 2024.5 Donors are formally advised if their ferritin result is outside the reference intervals of 15–400 μg/L for female donors and 30–500 μg/L for male donors.5 This will identify a considerable number of iron deficient adults who will be directed to their primary care physician for management. In the context of this policy change and implications for primary care, this article provides a guide for investigating and managing absolute iron deficiency.
Classifications of iron deficiency
Iron stores inadequate to meet the demands of the body result in absolute iron deficiency, which is associated with a compensatory reduction in serum hepcidin concentration to stimulate an increase in gastrointestinal iron absorption and restore homeostasis.6,7,8 Functional iron deficiency occurs when relatively normal iron stores are unable to be released for physiological requirements due to inappropriately elevated serum hepcidin levels, as may occur in chronic inflammatory conditions, including obesity, chronic disease and neoplasia.6,7,8 Absolute and functional iron deficiency can also co‐exist.7 A ferritin level below the reference interval should always be interpreted as absolute iron deficiency.
Diagnosis of iron deficiency
The diagnosis of iron deficiency is based on routinely available blood biomarkers as described in Box 1. Serum ferritin level cut‐offs to diagnose iron deficiency vary considerably,9 from less than 15 μg/L used by the World Health Organization,10 which predicts absent iron stores with very high specificity,7 to less than 30 μg/L commonly used in Australia.7,8,11,12 Although the sex‐based cut‐offs adopted by the Australian Red Cross Lifeblood were reportedly derived from the Royal College of Pathologists of Australasia,12 there is significant concern regarding inequalities using unconventional sex‐based cut‐offs, with underdiagnosis and undertreatment of iron deficient women. As an acute‐phase reactant, ferritin may be falsely normal or elevated in iron deficient individuals when there is concurrent inflammation, obesity, steatotic liver disease, malignancy or other chronic disease.6,7,11 Thus, a serum ferritin level less than 70 μg/L in an individual with a concurrent inflammatory condition may suggest iron deficiency.10 Transferrin saturation values less than 20% indicate low serum iron bioavailability, consistent with absolute or functional iron deficiency. The mean cell volume and mean cell haemoglobin concentration are sensitive measures for iron deficiency anaemia (IDA) when B12 or folate deficiency are absent.6,11
Causes and investigation of absolute iron deficiency
Further assessment must be conducted to define the underlying aetiology of iron deficiency, which can be broadly categorised into physiological, inadequate intake or absorption, blood loss, and rare genetic causes (Box 2). Factors such as age, sex and ethnicity influence the probability of different aetiologies. Although physiological causes are commonly suspected in younger individuals, the incidence of pathological causes, such as colorectal malignancy, increases with age (Box 3). Risk stratification is necessary to guide appropriate investigations. A suggested approach to a low serum ferritin result is presented in Box 4.
Physiological causes
Physiological causes of iron deficiency predominately affect pre‐menopausal women, pregnant women and growing children. Menstrual blood loss contributes significantly to iron deficiency, with iron deficiency affecting around half of women with heavy menstrual bleeding.6,7,8,11
Inadequate dietary intake or absorption
Iron uptake is affected by dietary intake and absorption. Haem iron is efficiently absorbed in the duodenum, whereas non‐haem iron is less efficiently absorbed and influenced by dietary composition.13 Several dietary factors inhibit non‐haem iron absorption (Box 2).13 The ingestion of ascorbic acid (vitamin C), lactic acid (found in fermented foods) and animal meat enhances the absorption of iron.13 Gastric acid, which can be impaired by medications, aids in the conversion of insoluble ferric non‐haem iron to the absorbable ferrous form.14 Surgical resection or bypass involving the stomach or small intestine (or both) predisposes the patient to iron deficiency due to malabsorption and reduced food intake.15
Coeliac disease has a global prevalence of 0.7–1.4% and accounts for about 3% of IDA in the general population.16 Serological testing for coeliac disease using serum tissue transglutaminase IgA antibody levels should be undertaken for assessment of iron deficiency.6,7 In the 2–3% of coeliac patients with IgA deficiency, an IgA level or IgG‐based assay, such as deamidated gliadin peptide, should be performed.6,7 Duodenal biopsies taken when the patient is on a gluten‐containing diet remains the gold standard for diagnosis of coeliac disease in Australia.
Autoimmune gastritis, generally associated with pernicious anaemia, is commonly refractory to oral iron supplementation and reported in 27% of individuals with unexplained IDA.17 Helicobacter pylori infection is associated with iron deficiency and eradication improves iron stores, especially in cases previously refractory to oral iron supplementation.17
In obesity, iron deficiency can result from reduced iron absorption.7
There are also rare genetic causes of unexplained iron deficiency.6,7
Blood loss
Blood loss is the most common and most significant pathological cause of iron deficiency, with aetiologies described in Box 2. Gastrointestinal blood loss accounts for most cases of iron deficiency,6,7,9,11,18 with bidirectional endoscopic examination detecting a potential gastrointestinal bleeding cause in two‐thirds of men and post‐menopausal women with IDA. Bidirectional endoscopic examination should be routinely considered for all adults with iron deficiency as the technique shows a detection rate of 11% for gastrointestinal malignancy and 9% for colorectal malignancy.6,7,8,9 Faecal occult blood testing is used for asymptomatic average‐risk bowel cancer screening and should not guide iron deficiency investigations.6,7 Small intestine investigation, predominantly with capsule endoscopy, may be considered in recurrent or refractory IDA following unremarkable bidirectional endoscopy results.19 When colonoscopy is contraindicated, computed tomography colonography can help rule out colorectal lesions larger than or equal to 6 mm, but cannot provide histopathological diagnosis.7,20 Medications can exacerbate blood loss, particularly non‐steroidal anti‐inflammatory drugs and anti‐thrombotics.6,7,8 Urinalysis is recommended to screen for microscopic haematuria, although this test is not sensitive for urological malignancies.6 Stool investigations for intestinal parasitic infections can be done for at‐risk individuals with exposure to endemic regions.8 Frequent whole blood donation can result in iron deficiency.11
Treatment
Iron supplementation, available in oral or parenteral forms, is required for all individuals with IDA and should be started promptly to replace body iron stores and resolve symptoms. Iron replacement for non‐anaemic iron deficiency improves symptoms and clinical outcomes.6,7 Iron‐deplete women with serum ferritin levels of 15–30 μg/L will not be identified through the Australian Red Cross Lifeblood testing, although may benefit from iron replacement. Blood transfusion with restrictive targets should be reserved for severe symptomatic anaemia and symptoms of reduced oxygen‐carrying capacity with cardiovascular compromise or ongoing blood loss, when iron replacement alone is insufficient.6 Increasing dietary iron intake alone is insufficient for iron deficiency, although should be optimised for secondary prevention.
Oral iron supplementation is considered first‐line therapy. Numerous formulations are available (Box 5). Multivitamins or supplements with low iron concentrations and modified‐release formulations are ineffective. Ferrous iron taken on an empty stomach ensures optimal absorption. Oral iron polymaltose is not dependent on gastric acidity or vitamin C, is best taken with food, and represents an alternative preparation.7 Elemental oral iron doses of larger than or equal to 60 mg reduce the absorption of subsequent oral doses.21 A single 200 mg dose taken on alternate days results in about twice the absorption of a single 100 mg daily dose.22 Twice‐daily dosing has the lowest absorption.23 Alternate day single doses are associated with significantly higher iron absorption and lower hepcidin induction compared with daily dosing,23 and may have reduced gastrointestinal adverse events,24 compliance concerns and therapy cessation.7
Supplementation with intravenous iron results in a faster haemoglobin concentration increase compared with oral iron supplementation, although haemoglobin levels at 12 weeks are similar.6,7,8,11 The indications, available formulations, duration of administration and maximum dosage are described in Box 6. Current parenteral iron formulations are well tolerated and suitable for administration in primary care. Monitoring is generally advised despite no associations with serious infusion reactions.25 Parenteral iron should be avoided in patients with active infection.25 Skin staining can occur with extravasation. Hypophosphataemia is most common after ferric carboxymaltose, with the condition usually asymptomatic, and recovery expected over eight to ten weeks.7 Routine measurement of phosphate is not recommended if asymptomatic. Intramuscular administration of iron is not routinely recommended.
Monitoring of response
After commencing iron therapy, haemoglobin levels should be checked within two to four weeks for an expected rise of more than or equal to 10 g/L.6 Serum ferritin and haemoglobin levels should be measured monthly for the first three months, targeting serum ferritin normalisation or a haemoglobin increase of 20 g/L in IDA.6 Oral iron should be continued for at least three months following an effective response; adherence and impaired iron uptake should be considered with inadequate responses. Thereafter, ferritin and haemoglobin levels should be monitored every three to six months for 12 months after treatment of iron deficiency and its underlying cause.6 Iron supplementation should be restarted if levels are not maintained, and further investigations considered.
Conclusion
Implementation of routine ferritin testing by the Australian Red Cross Lifeblood will increase detection of iron deficiency among blood donors. Low serum ferritin levels are diagnostic of absolute iron deficiency and require clinical evaluation. The aetiologies of iron deficiency can be broadly categorised into physiological, inadequate dietary intake or absorption, blood loss, and rare genetic causes. Risk stratification of individuals should be considered to target investigations. Following the diagnosis of iron deficiency, iron replacement should be started early.
Box 1 – The interpretation of readily available tests for assessment of iron deficiency7
|
|
Ferritin |
Transferrin saturation |
Haemoglobin |
Mean cell volume and mean cell haemoglobin concentration |
Reticulocyte haemoglobin content |
||||||||||
|
|
|||||||||||||||
|
Iron replete |
≥ 30 μg/L (men) and ≥ 15 μg/L (women)* |
> 20% |
Normal |
Normal |
Normal |
||||||||||
|
Absolute iron deficiency |
< 30 μg/L (men) and < 15 μg/L (women)* |
< 20% |
Normal or low (anaemia) |
Normal (early) or low |
Low |
||||||||||
|
Functional iron deficiency |
Normal or increased (inflammatory states) |
< 20% |
Mild to moderate anaemia |
Normal or mildly low |
Low |
||||||||||
|
Functional iron deficiency with absolute iron deficiency |
< 70–100 μg/L (influenced by degree of inflammation) |
< 20% |
Mild to moderate anaemia |
Reduced |
Low |
||||||||||
|
|
|||||||||||||||
|
*Based on serum ferritin reference intervals used by the Australian Red Cross Lifeblood. Women with serum ferritin levels of 15–30 μg/L may also have iron deficiency and will not be detected and referred from the Australian Red Cross Lifeblood service. |
|||||||||||||||
Box 2 – Aetiologies and their likelihood (in brackets) as a cause of absolute iron deficiency
|
Aetiology |
Examples |
||||||||||||||
|
|
|||||||||||||||
|
Physiological (very common) |
Menstruation, pregnancy, lactation, growth |
||||||||||||||
|
Inadequate intake (common) |
Diet |
||||||||||||||
|
Abnormal absorption (common) |
|
||||||||||||||
|
Blood loss (common) |
|
||||||||||||||
|
Genetic (rare and not easily diagnosed) |
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – Common aetiologies of iron deficiency in adults broadly grouped into physiological, inadequate intake, blood loss, and abnormal absorption, with a visual representation of estimated likelihoods across the age spectrum
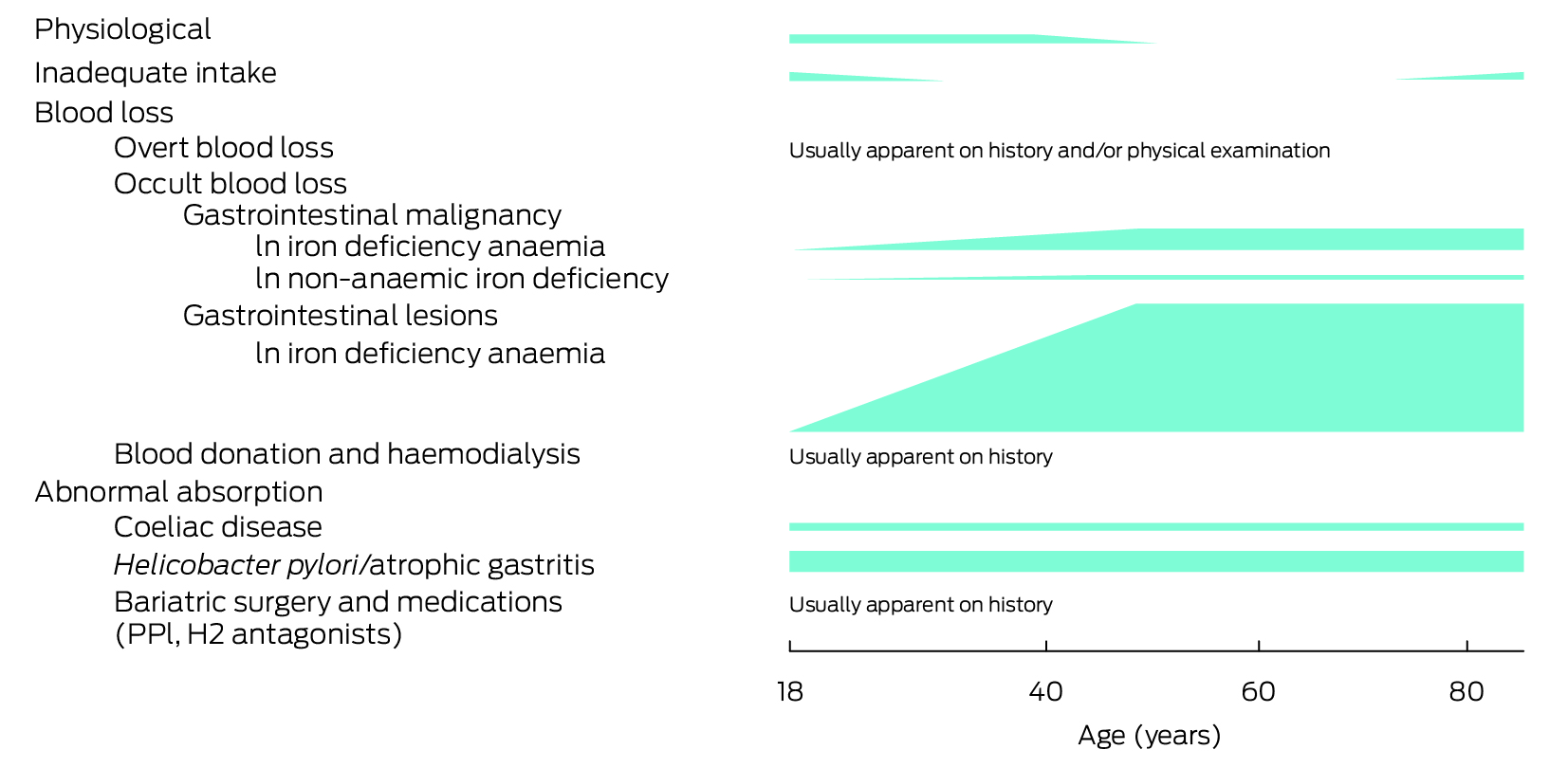
H2 = histamine type‐2 receptor; PPI = proton pump inhibitor. Likelihoods are estimations and, where available, based on published data predominantly in iron deficiency anaemia.3,6,7,8,9,17,18
Box 4 – A general approach to assessment, management and investigation of a low serum ferritin level
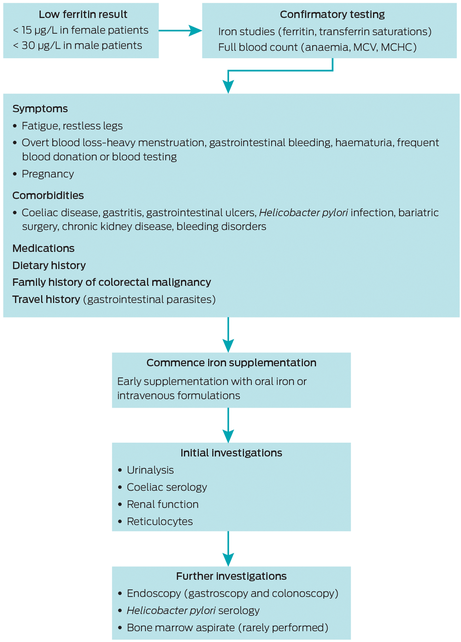
MCHC = mean cell haemoglobin concentration; MCV = mean cell volume.
Box 5 – Formulations of oral iron therapies in Australia
|
Brand name |
Formulation |
Elemental iron content |
|||||||||||||
|
|
|||||||||||||||
|
Ferro‐tab* |
|
65.7 mg |
|||||||||||||
|
Ferro‐F‐tab* |
|
100 mg |
|||||||||||||
|
Ferro‐liquid* |
|
6 mg/mL |
|||||||||||||
|
Ferro‐Gradumet |
|
105 mg |
|||||||||||||
|
Ferrograd C |
|
105 mg |
|||||||||||||
|
Maltofer |
|
100 mg |
|||||||||||||
|
FGF† |
|
80 mg |
|||||||||||||
|
Fefol† |
|
87 mg |
|||||||||||||
|
|
|||||||||||||||
|
* Pharmaceuticals Benefits Scheme. † Ferrous preparations with folic acid are designed for treatment during pregnancy. In anaemic subjects, serum iron, B12 and folate levels should be tested and if subjects are folate deficient, a therapeutic dose of 5 mg daily prescribed. Administration of folic acid combined with iron but without checking folate or B12 levels may precipitate subacute combined degeneration of spinal cord in patients who are B12 deficient. |
|||||||||||||||
Box 6 – Intravenous formulations of iron available on the Pharmaceuticals Benefits Scheme in Australia
|
Brand name |
Compound |
Formulation |
Maximum single dose |
Duration of infusion |
|||||||||||
|
|
|||||||||||||||
|
Ferinject |
Ferric carboxymaltose |
500 mg/10 mL vial or 1 g/20 mL vial |
1000 mg, repeat a week later |
Up to 15 minutes depending on dose |
|||||||||||
|
Ferrosig* |
Iron polymaltose |
100 mg/2 mL ampoule |
1000–2500 mg |
60–120 minutes |
|||||||||||
|
Venofer* |
Iron sucrose |
100 mg/5 mL ampoule |
100 mg during dialysis 3 times per week |
15 minutes minimum |
|||||||||||
|
Monofer |
Ferric derisomaltose |
500 mg/5 mL vial |
1500 mg |
30 minutes |
|||||||||||
|
|
|||||||||||||||
|
PBS = Pharmaceutical Benefits Scheme. * Streamline PBS Authority for iron deficiency anaemia while undergoing chronic haemodialysis. Intravenous iron can be considered for: individuals with contraindications or intolerances to oral iron, pregnancy (after the first trimester), postpartum state, chronic kidney disease on erythropoiesis‐stimulating therapies, conditions reducing oral iron absorption, iron losses exceeding absorption capacity, requiring rapid iron replacement, and haemoglobin not rising by 10 g/L within two weeks of commencing oral iron.6,7,8,11 |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993‐2005. Public Health Nutr 2009; 12: 444‐454.
- 2. GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990‐2021: findings from the Global Burden of Disease Study 2021. Lancet Haematol 2023; 10: e713‐e734.
- 3. Australian Bureau of Statistics. Australian health survey: biomedical results for nutrients. Canberra: ABS, 2013. https://www.abs.gov.au/statistics/health/health‐conditions‐and‐risks/australian‐health‐survey‐biomedical‐results‐nutrients/latest‐release (viewed Sept 2023).
- 4. Australian Red Cross Lifeblood. Lifeblood stats and snacks 2023 [website]. Australia, 2023. https://www.lifeblood.com.au/news‐and‐stories/vital‐reads/2023‐stats‐and‐snacks (viewed Apr 2024).
- 5. Australian Red Cross Lifeblood. Ferritin (iron) testing [website]. Australia, 2023. https://www.lifeblood.com.au/blood/learn‐about‐blood/iron‐health/ferritin‐testing (viewed Oct 2023).
- 6. Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021; 70: 2030‐2051.
- 7. Pasricha SR, Tye‐Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet 2021; 397: 233‐248.
- 8. Gastroenterological Society of Australia (GESA). Clinical update for general practitioners and physicians: iron deficiency. Melbourne: GESA, 2022. https://www.gesa.org.au/public/13/files/Education%20&%20Resources/Clinical%20Practice%20Resources/Iron%20Deficiency/Iron%20Deficiency%20Clinical%20Update%202022%20APPROVED.pdf (viewed Feb 2024).
- 9. Rockey DC, Altayar O, Falck‐Ytter Y, Kalmaz D. AGA technical review on gastrointestinal evaluation of iron deficiency anemia. Gastroenterology 2020; 159: 1097‐1119.
- 10. World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Geneva: WHO, 2020. https://www.who.int/publications/i/item/9789240000124 (viewed Oct 2023).
- 11. Low MS, Grigoriadis G. Iron deficiency and new insights into therapy. Med J Aust 2017; 207: 81‐87. https://www.mja.com.au/journal/2017/207/2/iron‐deficiency‐and‐new‐insights‐therapy
- 12. The Royal College of Pathologists of Australasia. Iron studies standardised reporting protocol. Second edition: November 2021. Sydney: RCPA, 2021. https://www.rcpa.edu.au/getattachment/554ba672‐4d34‐4e7c‐b812‐5741359bca78/Iron‐Studies‐Standardised‐Reporting‐Protocol.aspx (viewed Apr 2024).
- 13. Saunders AV, Craig WJ, Baines SK, Posen JS. Iron and vegetarian diets. Med J Aust 2013; 199 Suppl 4: S11‐16. https://www.mja.com.au/journal/2013/199/4/iron‐and‐vegetarian‐diets
- 14. Lam JR, Schneider JL, Quesenberry CP, Corley DA. Proton pump inhibitor and histamine‐2 receptor antagonist use and iron deficiency. Gastroenterology 2017; 152: 821‐829.
- 15. Gowanlock Z, Lezhanska A, Conroy M, et al. Iron deficiency following bariatric surgery: a retrospective cohort study. Blood Adv 2020; 4: 3639‐3647.
- 16. Mahadev S, Laszkowska M, Sundström J, et al. Prevalence of celiac disease in patients with iron deficiency anemia ‐ a systematic review with meta‐analysis. Gastroenterology 2018; 155: 374‐382.
- 17. Hershko C, Hoffbrand AV, Keret D, et al. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica 2005; 90: 585‐595.
- 18. Alexandre L, Manning C, Chan SSM. Prevalence of gastrointestinal malignancy in iron deficiency without anaemia: a systematic review and meta‐analysis. Eur J Intern Med 2020; 72: 27‐33.
- 19. Pennazio M, Spada C, Eliakim R, et al. Small‐bowel capsule endoscopy and device‐assisted enteroscopy for diagnosis and treatment of small‐bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47: 352‐376.
- 20. Atkin W, Dadswell E, Wooldrage K, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet 2013; 381: 1194‐1202.
- 21. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice‐daily doses in iron‐depleted young women. Blood 2015; 126: 1981‐1989.
- 22. Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron‐deficient anemic women. Haematologica 2020; 105: 1232‐1239.
- 23. Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice‐daily split dosing in iron‐depleted women: two open‐label, randomised controlled trials. Lancet Haematol 2017; 4: e524‐e533.
- 24. Kaundal R, Bhatia P, Jain A, et al. Randomized controlled trial of twice‐daily versus alternate‐day oral iron therapy in the treatment of iron‐deficiency anemia. Ann Hematol 2020; 99: 57‐63.
- 25. Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc 2015; 90: 12‐23.






Open access:
Open access publishing facilitated by Curtin University, as part of the Wiley ‐ Curtin University agreement via the Council of Australian University Librarians.
No relevant disclosures.