The known: Medications assigned to category X by the Therapeutic Goods Administration should be used by women of reproductive age only if they are also using reliable contraception.
The new: The prevalence of category X medication dispensing to women of reproductive age increased in Australia during 2013–2021, but rates of concurrent hormonal contraception were low (long‐acting reversible contraceptives, 13.2%; any hormonal contraception, 22.1%).
The implications: Low rates of highly effective hormonal contraceptive overlap among women dispensed teratogenic medications raises concerns about potential fetal harm during unintended pregnancies. Increasing the awareness and uptake of hormonal contraception by women prescribed teratogenic medications is needed.
Teratogens are substances, including medications, that can cause irreversible harm to the fetus if used during pregnancy.1 It is important to ensure that teratogens are used safely by women of childbearing age, including by reducing the risk of exposure during pregnancy. Regulatory agencies play a key role in assessing medications for their potential effects on the fetus. In Australia, the Therapeutic Goods Administration categorisation system for prescribing medicines in pregnancy includes major teratogens in categories D and X;2 category X is reserved for medications contraindicated during pregnancy because of the unacceptably high risk of fetal harm.2 Recognised teratogens include isotretinoin3 and thalidomide,4 which cause birth defects in 35–50% of exposed babies.
Guidelines recommend that women of reproductive age prescribed teratogenic medications use highly effective contraception methods to avoid unintended pregnancy.5 Long‐acting reversible contraceptives (LARCs) are the most effective reversible methods, including the copper intrauterine device, the levonorgestrel intrauterine system, and the progestogen‐only implant, each with failure rates lower than 1%.6 As the failure rates for methods such as contraceptive injection and contraceptive pills are 6–9%, additional contraceptive precautions (eg, condoms) are advised for women using these methods while taking teratogenic medications.6 Wide variation in the proportion of women using effective contraception while taking teratogenic medications has been reported (1.7–100%).7 Only limited data on the frequency of teratogenic medication use, and whether recommendations regarding concurrent contraception are followed, have been reported.
The objective of this study was to examine patterns in the dispensing of category X medications to women aged 15–49 years in Australia during 2008–2021, and patterns of concurrent use of hormonal LARC and other hormonal contraception.
Methods
For our retrospective, longitudinal cohort study, we analysed data in the Pharmaceutical Benefits Scheme (PBS) 10% sample dataset for 1 January 2008 – 31 December 2021. The dataset, a national, longitudinal, individual‐level extract of all PBS dispensing data for a random 10% sample of Australians eligible for PBS‐subsidised medicines, has been widely used for drug use research.8 The PBS subsidises a broad range of medications for Australian citizens, permanent residents, and citizens of countries with reciprocal health care agreements.9 We selected the 2013 calendar year as the first year of our analysis of exposure to prescribed teratogens, providing a 5‐year look back period to 2008 for identifying prior contraceptive dispensing.
The PBS dataset includes the PBS item code, medication strength, dispensed quantity, date of prescribing, date of supply, and state or territory of supply for each dispensed medication, as well as demographic information for the person dispensed the medication (year of birth, sex, year of death, concessional health care card status). The dataset does not include data on private prescriptions funded by the recipient or a private health insurer, inpatient prescriptions in public hospitals, or certain types of contraception not included in the PBS (eg, copper intrauterine devices).
Study population and exposure
We included women aged 15–49 years dispensed a category X medication at least once during 2013–2021 (Supporting Information, table 1).
Outcomes
We defined incident dispensing as the dispensing of a category X medication to a woman not dispensed the same medication during the preceding twelve months, and prevalent dispensing as the dispensing of a category X medication during a given calendar year. We calculated incidence and prevalence rates (per 1000 women) using Australian Bureau of Statistics population data for women aged 15–49 years in each state and territory.10
Contraceptive overlap was determined for incident users by examining the dispensing history of each woman. LARC overlap was defined as the dispensing of LARC prior to the category X medication, and the anticipated duration of contraceptive efficacy (five years for hormonal intrauterine devices, three years for implants) overlapping the category X medication dispensing date (Supporting Information, figure 1 and table 2). Similarly, non‐LARC hormonal contraceptive overlap was defined as the dispensing of a non‐LARC contraceptive prior to the category X medication, and the anticipated duration of contraceptive efficacy (112 days for the combined oral contraceptive and progestogen‐only pills, 84 days for injections) overlapping the category X medication dispensing date. Women with both LARC and non‐LARC hormonal contraceptive overlap (ie, possible use of multiple contraceptive methods) were classified as having LARC overlap.
In a sensitivity analysis, contraceptive dispensing was deemed to be overlapping if dispensed prior to or within 60 days of the dispensing of a category X medication.
For women dispensed the same category X medication two or more times, we examined contraceptive overlap at the time of each dispensing, and the longitudinal assessments of overlap were subsequently categorised as always, sometimes, or never overlapping.
Covariates
Data were available for age and concessional health care card status at the time of medication dispensing, as well as the state or territory where the medication was dispensed.
Statistical analysis
We summarise data as frequencies and proportions. We evaluated the statistical significance of differences by selected characteristics in the likelihood of contraceptive overlap in multivariable logistic regression analyses; we report adjusted odds ratios (aORs) with 95% confidence intervals (CIs). Change in contraceptive overlap over time was assessed by including calendar year as a linear term in the regression model. Analyses were undertaken in Stata MP 17.
Ethics approval
This study was approved by the Monash University Human Research Ethics Committee (ID 22877). The study protocol was approved and the final manuscript noted by the Services Australia External Requests Evaluation Committee (RMS0201).
Results
We identified 15 627 incident users of category X medications during 2013–2021 (Box 1).
Change in category X medication dispensing, 2013–2021
The incidence of category X medication dispensing increased from 2.3 per 1000 women aged 15–49 years in 2013 to 4.0 per 1000 women aged 15–49 years in 2021. The most frequently dispensed class of category X medications were dermatological agents, the only class for which incident dispensing increased during this period (from 2.1 to 3.8 per 1000 women aged 15–49 years) (Box 2).
The prevalence of category X medication dispensing increased from 4.6 per 1000 women aged 15–49 years in 2013 to 8.7 per 1000 women aged 15–49 years in 2021. The largest increase in dispensing prevalence was for dermatological agents, rising from 3.9 to 7.9 per 1000 women aged 15–49 years (Box 2).
Contraceptive overlap at first dispensing of category X medications
LARC overlap was inferred for 2059 of 15 627 women at the time of the first dispensing of category X medications (13.2%); 3441 had been dispensed any type of hormonal contraception (22.1%). The proportion of women with LARC overlap was smallest for those dispensed dermatological agents (1806 of 14 331 women, 12.6%) (Box 3).
Among women first dispensed dermatological agents, LARC overlap (aOR, 0.17; 95% CI, 0.14–0.20) and any hormonal contraception overlap (aOR, 0.28; 95% CI, 0.25–0.32) were less likely for those aged 15–19 years than for women aged 25–29 years. LARC overlap (aOR, 1.29; 95% CI, 1.14–1.46) and any hormonal contraceptive overlap (aOR, 1.46; 95% CI, 1.21–1.48) were more likely for women with than those without health care concession cards. The likelihood of LARC or any hormonal contraceptive overlap also varied by state and territory. The LARC overlap proportion increased from 116 of 1151 (10.1%) in 2013 to 343 of 2260 (15.2%) in 2021 (Box 4). For women first dispensed anti‐neoplastic or immunomodulating agents, similar differences in LARC overlap by age, state/territory, and year were noted (with much smaller dispensing numbers), but the difference by concessional health card status was not statistically significant (Box 5).
In the sensitivity analysis (contraception commenced up to 60 days after first dispensing of category X medication), the LARC overlap proportion increased slightly, from 13.2% to 13.6% (2122 of 15 627 women) (Supporting Information, table 5).
Contraceptive overlap at time of each dispensing
Of 14 115 women dispensed the same category X medication two or more times during 2013–2021, LARC overlap at the time of each dispensing was inferred for 1568 (11.5%), and sometimes for 710 (5.2%). Any hormonal contraceptive overlap at the time of each dispensing was inferred for 2198 women (16.1%), and sometimes for 2042 (14.9%). The LARC overlap proportion at the time of every dispensing was smallest for those dispensed dermatological medications (1391 of 12 597, 11.0%) (Box 6). Age group, health care concessional card status, state/territory, and year each influenced the likelihood of LARC overlap or any hormonal contraception overlap at the time of each dispensing of a dermatological category X medication (Supporting Information, table 7), but only year influenced the likelihood of LARC overlap or any hormonal contraception overlap at the time of each dispensing of antineoplastic and immunomodulating agents (Supporting Information, table 8).
Discussion
In our population‐based study, we found that both incident and prevalent teratogenic medication dispensing to women aged 15–49 years almost doubled in Australia between 2013 and 2021, and that hormonal LARC dispensing overlapping the first dispensing of teratogenic medications was recorded in only 13.2% of cases. Our findings of increasing use of teratogenic medications and low rates of hormonal LARC overlap raise concerns about the risk of unintended pregnancy and potentially preventable fetal harm from teratogenic medications, particularly in pregnant women under 25 years of age.
Data on the number of unintended pregnancies in women taking category X medications are limited. The Therapeutic Goods Administrations database of adverse event notifications includes at least 72 cases of isotretinoin exposure during pregnancy during 1985–2023.11 An analysis of pregnancy terminations in South Australia during 1985–1993 identified that fifteen involved exposure to isotretinoin.12 Further, a study of phone calls during 2010–2014 to MotherSafe, a NSW medications in pregnancy and lactation advisory service, identified 49 related to isotretinoin exposure during pregnancy.13 During 2005–2012, the prevalence in New South Wales of category X medication use in the year preceding pregnancy (total of 191 588 pregnancies) ranged between 0.01% and 0.06%;14 the study, however, did not include pregnancies ending in terminations, probably leading to underestimation of the prevalence of teratogen exposure.
A systematic review of risk management when prescribing teratogenic medications to women of childbearing age identified 55 studies, 36 of which reported contraceptive use during treatment with teratogenic medications; the proportions of women using contraception before starting treatment (15.7% to 94%) or during treatment (1.7% to 100%) each varied widely between studies.7 The only two studies based on national dispensing data, both undertaken in the Netherlands, found that contraceptive overlap ranged from 23% to 64%, depending on the teratogenic medication type;15,16 the prevalence of hormonal contraceptive overlap in our study was 22%. The reasons for the low degree of contraceptive overlap are unknown and require investigation.
Despite recognition that they are among the most effective contraceptive methods, LARC dispensing to women prescribed category X medications (13.2%) is only marginally greater than the estimated overall rate of LARC use in Australia (10.8%).17 The low and variable rates of LARC overlap we found suggest that barriers to LARC access for women using category X medications need to be overcome, including poor access and awareness, misconceptions about its suitability (among both women and providers), costs, and concerns about side effects.18 While LARC dispensing costs are subsidised by the PBS, out‐of‐pocket costs for insertion can be considerable. Further, as most category X medication dispensing is of isotretinoin, concerns about the side effects of contraception could be the greatest barrier; progestogen‐containing LARC can exacerbate acne in about one‐third of users.19
The suboptimal rates of concurrent contraception dispensing raise questions about the effectiveness of measures for reducing the potential harms of category X medication use during pregnancy. Risk management measures vary considerably, both in Australia and overseas. A detailed risk management program for thalidomide prescribing requires online registration of women for whom it has been prescribed and a negative pregnancy test result at the time of treatment initiation.20 For other category X medications, informal measures, such as warnings in the product labelling or information leaflets, are used without rigorous monitoring. There is no formal risk management program for isotretinoin in Australia, although such programs have been established overseas,7 and isotretinoin causes major untreatable and lifelong birth defects in as many as 35% of exposed babies. Instead, isotretinoin prescribing is restricted to dermatologists, the assumption being that a smaller group of specialist prescribers is better placed to discuss fetal risks and ensure adherence to recommendations about contraception. However, the Royal Australian College of General Practitioners called in 2014 for prescribing responsibilities to be expanded to general practitioners, well placed to provide access to a range of contraceptive methods, as many are trained in LARC insertion or can better facilitate access to LARC insertion.21 In the Netherlands where isotretinoin prescribing is not restricted to dermatologists, contraceptive overlap in 2012 was slightly higher among those prescribed isotretinoin by general practitioners than by specialists (71% v 63%).15 In New Zealand, general practitioners have prescribed isotretinoin since 2009, and rates of unintended pregnancies and terminations did not change in the subsequent eight years.22
Irrespective of prescribing restrictions, our findings suggest that the uptake of highly effective contraceptive methods by Australian women prescribed category X medications could be improved. Effective strategies for increasing LARC uptake in primary care include better training in contraceptive counselling and improving access to rapid referral clinics for insertion.23 These approaches could be adapted for use by other specialists, including dermatologists, accompanied by education of women prescribed category X medications. Ongoing surveillance of concurrent contraceptive use, as well as of unintended pregnancies and their outcomes, is also critical for refining risk management.
Limitations
As our analysis was restricted to PBS‐subsidised medications, data for certain combined oral contraceptive pills, vaginal rings, and copper intrauterine devices were not available, nor for male and female sterilisation. Recent Australian studies of contraception in the general population found that 5–10% of women used contraceptives not subsidised by the PBS;23,24 we may therefore have underestimated absolute hormonal contraceptive overlap. Further, the outcomes data analysed were dispensing data rather than data on the duration of contraception use.
Conclusion
Contraceptive overlap using highly effective hormonal contraceptive methods is low among women dispensed category X medications in Australia. Why LARC uptake is low should be investigated, and strategies developed to improve the use of highly effective contraception, particularly by women aged 15–19 years prescribed category X dermatological medications.
Box 1 – Characteristics of 15 627 women of reproductive age (15–49 years), Australia, dispensed category X medications during 2013–2021 (incident users)
|
Characteristic |
Number |
||||||||||||||
|
|
|||||||||||||||
|
Medication class (ATC level 1) |
|
||||||||||||||
|
(D) Dermatological agents |
14 331 (91.7%) |
||||||||||||||
|
(L) Anti‐neoplastic and immunomodulating agents |
1214 (7.8%) |
||||||||||||||
|
(C) Cardiovascular system agents |
55 (0.4%) |
||||||||||||||
|
(J) Anti‐infectives for systemic use |
27 (0.2%) |
||||||||||||||
|
Medication name |
|
||||||||||||||
|
Isotretinoin |
14 102 (90.2%) |
||||||||||||||
|
Leflunomide |
1037 (6.6%) |
||||||||||||||
|
Acitretin |
229 (1.5%) |
||||||||||||||
|
Teriflunomide |
129 (0.8%) |
||||||||||||||
|
Macitentan |
37 (0.2%) |
||||||||||||||
|
Ribavirin |
26 (0.2%) |
||||||||||||||
|
Thalidomide |
17 (0.1%) |
||||||||||||||
|
Arsenic |
11 (0.1%) |
||||||||||||||
|
Lenalidomide |
10 (0.1%) |
||||||||||||||
|
Other* |
29 (0.2%) |
||||||||||||||
|
Age group at first dispensing (years) |
|
||||||||||||||
|
15–19 |
5893 (37.7%) |
||||||||||||||
|
20–24 |
3507 (22.4%) |
||||||||||||||
|
25–29 |
2215 (14.2%) |
||||||||||||||
|
30–34 |
1149 (7.4%) |
||||||||||||||
|
35–39 |
948 (6.1%) |
||||||||||||||
|
40–44 |
925 (5.9%) |
||||||||||||||
|
45–49 |
990 (6.3%) |
||||||||||||||
|
State/territory |
|
||||||||||||||
|
Australian Capital Territory |
320 (2.0%) |
||||||||||||||
|
New South Wales |
4926 (31.5%) |
||||||||||||||
|
Northern Territory |
44 (0.3%) |
||||||||||||||
|
Queensland |
3281 (21.0%) |
||||||||||||||
|
South Australia |
970 (6.2%) |
||||||||||||||
|
Tasmania |
297 (1.9%) |
||||||||||||||
|
Victoria |
4084 (26.1%) |
||||||||||||||
|
Western Australia |
1705 (10.9%) |
||||||||||||||
|
Health care concession card status |
|
||||||||||||||
|
No concession card |
12 489 (80.0%) |
||||||||||||||
|
Concession card holder |
3122 (20.0%) |
||||||||||||||
|
Missing data |
16 |
||||||||||||||
|
|
|||||||||||||||
|
ATC = Anatomical Therapeutic Chemical (ATC) classification system. * Bosentan, ambrisentan, riociguat, azacitidine, sonidegib, and pomalidomide were each dispensed to fewer than ten women during the study period. |
|||||||||||||||
Box 2 – Incident and prevalent dispensing of category X medications to women aged 15–49 years, Australia, 2013–2021, overall and by Anatomical Therapeutic Chemical (ATC) level 1 category
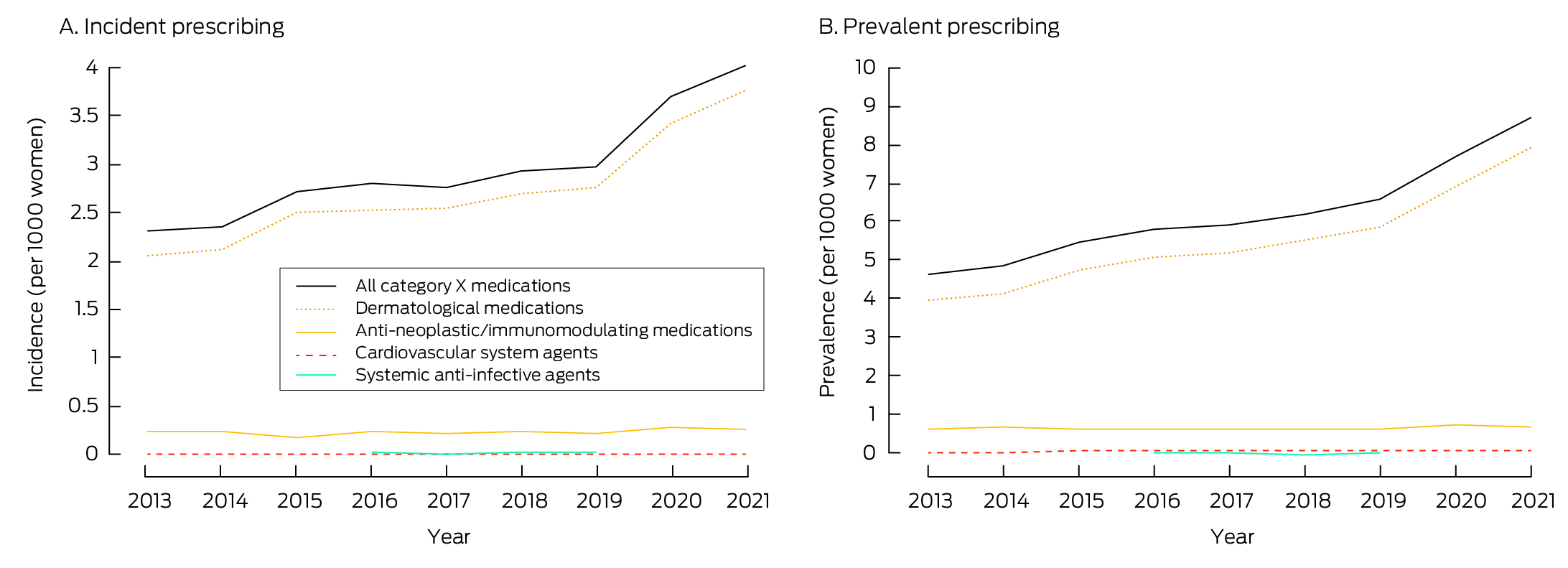
The data underlying these graphs are available in the Supporting Information, table 3.
Box 3 – Contraceptive overlap at the time of first dispensing of category X medications, Australia, 2013–2021, by Anatomical Therapeutic Chemical (ATC) level 1 category
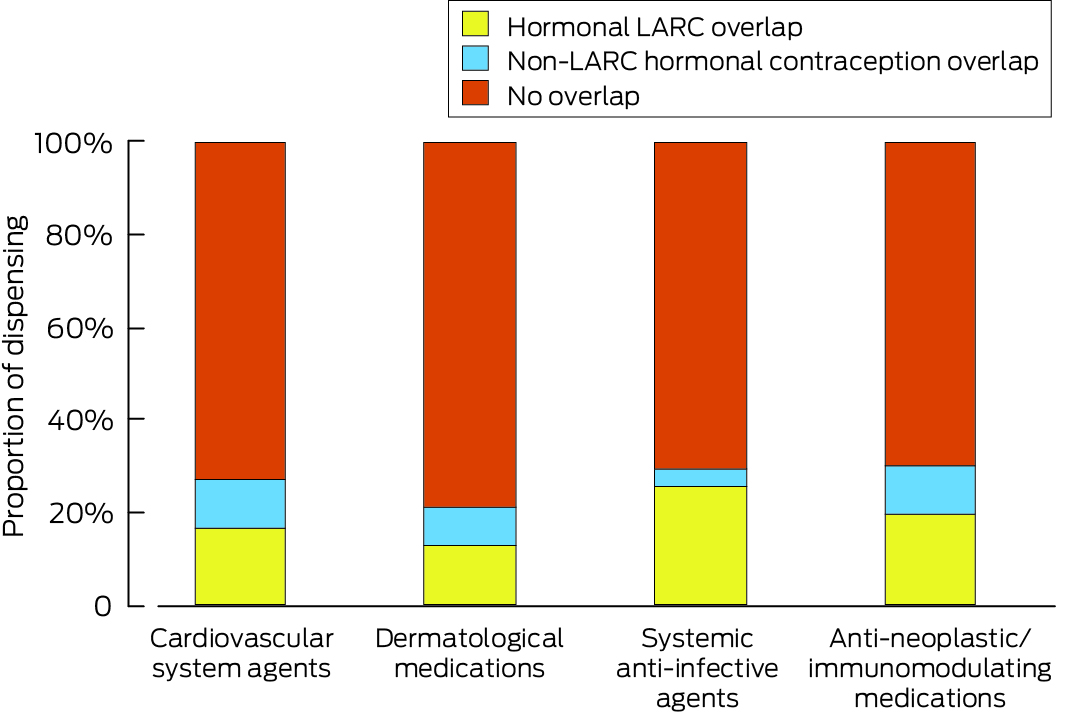
LARC = long‐acting reversible contraceptives.The data underlying these graphs are available in the Supporting Information, table 4.
Box 4 – Contraceptive overlap at the time of first dispensing of category X dermatological agents to women aged 15–49 years, Australia, 2013–2021: multivariable logistic regression analysis*
|
|
Hormonal LARC overlap |
Any hormonal contraceptive overlap |
|||||||||||||
|
Characteristic |
Number |
aOR (95% CI) |
Number |
aOR (95% CI) |
|||||||||||
|
|
|||||||||||||||
|
Age group (years) |
|
|
|
|
|||||||||||
|
15–19 |
232/5868 |
0.17 (0.14–0.20) |
635/5868 |
0.28 (0.25–0.32) |
|||||||||||
|
20–24 |
600/3464 |
0.84 (0.73–0.97) |
1020/3464 |
0.95 (0.85–1.09) |
|||||||||||
|
25–29 |
409/2133 |
1 |
633/2133 |
1 |
|||||||||||
|
30–34 |
209/1051 |
1.02 (0.85–1.23) |
292/1051 |
0.89 (0.76–1.05) |
|||||||||||
|
35–39 |
154/726 |
1.12 (0.91–1.38) |
203/726 |
0.90 (0.75–1.09) |
|||||||||||
|
40–44 |
117/595 |
1.03 (0.81–1.29) |
153/595 |
0.81 (0.66–1.00) |
|||||||||||
|
45–49 |
84/494 |
0.85 (0.65–1.10) |
110/494 |
0.66 (0.52–0.83) |
|||||||||||
|
Health care concession a card status |
|
|
|
|
|||||||||||
|
No concession card |
1390/11557 |
1 |
2337/11 557 |
1 |
|||||||||||
|
Concession card holder |
414/2766 |
1.29 (1.14–1.46) |
708/2766 |
1.46 (1.21–1.48) |
|||||||||||
|
State/territory |
|
|
|
|
|||||||||||
|
New South Wales |
415/4517 |
1 |
735/4517 |
1 |
|||||||||||
|
Australian Capital Territory |
56/279 |
2.50 (1.81–3.46) |
73/279 |
1.81 (1.36–2.41) |
|||||||||||
|
Northern Territory |
10/40 |
3.18 (1.60–6.79) |
13/40 |
2.34 (1.18–4.65) |
|||||||||||
|
Queensland |
431/3026 |
1.66 (1.43–1.93) |
677/3026 |
1.51 (1.34–1.70) |
|||||||||||
|
South Australia |
157/887 |
2.07 (1.68–2.55) |
249/887 |
1.97 (1.66–2.34) |
|||||||||||
|
Tasmania |
61/247 |
3.48 (2.51–4.81) |
94/247 |
3.37 (2.54–4.46) |
|||||||||||
|
Victoria |
471/3825 |
1.30 (1.13–1.50) |
862/3825 |
1.44 (1.28–1.61) |
|||||||||||
|
Western Australia |
204/1510 |
1.58 (1.32–1.90) |
343/1510 |
1.54 (1.33–1.79) |
|||||||||||
|
Year |
|
|
|
|
|||||||||||
|
2013 |
116/1151 |
1 |
221/1151 |
1 |
|||||||||||
|
2014 |
131/1190 |
1.10 (0.84–1.44) |
247/1190 |
1.08 (0.88–1.34) |
|||||||||||
|
2015 |
168/1432 |
1.18 (0.91–1.53) |
298/1432 |
1.10 (0.90–1.34) |
|||||||||||
|
2016 |
174/1459 |
1.23 (0.96–1.59) |
298/1459 |
1.08 (0.89–1.32) |
|||||||||||
|
2017 |
181/1495 |
1.30 (1.01–1.68) |
292/1495 |
1.05 (0.86–1.29) |
|||||||||||
|
2018 |
195/1603 |
1.34 (1.04–1.72) |
331/1603 |
1.15 (0.94–1.39) |
|||||||||||
|
2019 |
221/1661 |
1.52 (1.19–1.94) |
386/1661 |
1.37 (1.13–1.66) |
|||||||||||
|
2020 |
276/2080 |
1.50 (1.18–1.90) |
443/2080 |
1.20 (1.00–1.45) |
|||||||||||
|
2021 |
343/2260 |
1.72 (1.37–2.17) |
530/2260 |
1.35 (1.13–1.62) |
|||||||||||
|
|
|||||||||||||||
|
aOR = adjusted odds ratio; CI = confidence interval; LARC = long‐acting reversible contraception. * Adjusted for age group, concessional status, state/territory, and calendar year. |
|||||||||||||||
Box 5 – Contraceptive overlap at the time of first dispensing of category X antineoplastic and immunomodulating agents to women aged 15–49 years, Australia, 2013–2021: multivariable logistic regression analysis*
|
|
Hormonal LARC overlap |
Any hormonal contraceptive overlap |
|||||||||||||
|
Characteristic |
Number |
aOR (95% CI) |
Number |
aOR (95% CI) |
|||||||||||
|
|
|||||||||||||||
|
Age group (years) |
|
|
|
|
|||||||||||
|
15–19 |
1/22 |
0.14 (0.02–1.18) |
8/22 |
0.71 (0.26–1.93) |
|||||||||||
|
20–24 |
8/37 |
0.83 (0.31–2.24) |
14/37 |
0.71 (0.31–1.61) |
|||||||||||
|
25–29 |
17/80 |
1 |
34/80 |
1 |
|||||||||||
|
30–34 |
27/89 |
1.49 (0.72–3.08) |
40/89 |
1.05 (0.57–1.96) |
|||||||||||
|
35–39 |
41/212 |
0.82 (0.43–1.59) |
68/212 |
0.61 (0.36–1.05) |
|||||||||||
|
40–44 |
60/313 |
0.79 (0.42–1.48) |
88/313 |
0.50 (0.30–0.84) |
|||||||||||
|
45–49 |
83/461 |
0.71 (0.38–1.30) |
117/461 |
0.41 (0.25–0.68) |
|||||||||||
|
Health care concession card status |
|
|
|
|
|||||||||||
|
No concession card |
157/826 |
1 |
240/826 |
1 |
|||||||||||
|
Concession card holder |
80/386 |
1.17 (0.85–1.60) |
129/386 |
1.22 (0.93–1.59) |
|||||||||||
|
State/territory |
|
|
|
|
|||||||||||
|
New South Wales |
69/382 |
1 |
112/382 |
1 |
|||||||||||
|
Australian Capital Territory |
14/36 |
2.81 (1.34–5.89) |
15/36 |
1.71 (0.84–3.49) |
|||||||||||
|
Northern Territory |
NR |
NR |
NR |
NR |
|||||||||||
|
Queensland |
49/239 |
1.15 (0.76–1.75) |
73/239 |
1.08 (0.75–1.55) |
|||||||||||
|
South Australia |
19/79 |
1.49 (0.83–2.70) |
29/79 |
1.44 (0.86–2.41) |
|||||||||||
|
Tasmania |
13/43 |
1.94 (0.94–4.02) |
19/43 |
1.98 (1.02–3.84) |
|||||||||||
|
Victoria |
36/249 |
0.84 (0.54–1.32) |
66/249 |
0.91 (0.63–1.31) |
|||||||||||
|
Western Australia |
36/184 |
1.21 (0.76–1.93) |
53/184 |
1.01 (0.68–1.50) |
|||||||||||
|
Year |
|
|
|
|
|||||||||||
|
2013 |
17/134 |
1 |
34/134 |
1 |
|||||||||||
|
2014 |
13/130 |
0.79 (0.36–1.71) |
35/130 |
1.12 (0.64–1.96) |
|||||||||||
|
2015 |
17/105 |
1.37 (0.65–2.89) |
28/105 |
1.12 (0.61–2.04) |
|||||||||||
|
2016 |
25/136 |
1.59 (0.81–3.13) |
39/136 |
1.23 (0.71–2.13) |
|||||||||||
|
2017 |
17/122 |
1.17 (0.56–2.43) |
30/122 |
0.98 (0.55–1.76) |
|||||||||||
|
2018 |
28/142 |
1.75 (0.90–3.40) |
44/142 |
1.36 (0.79–2.33) |
|||||||||||
|
2019 |
28/130 |
2.02 (1.04–3.95) |
44/130 |
1.61 (0.93–2.78) |
|||||||||||
|
2020 |
56/165 |
3.55 (1.92–6.56) |
67/165 |
2.06 (1.24–3.45) |
|||||||||||
|
2021 |
36/150 |
2.32 (1.22–4.41) |
48/150 |
1.45 (0.85–2.47) |
|||||||||||
|
|
|||||||||||||||
|
aOR = adjusted odds ratio; CI = confidence interval; LARC = long‐acting reversible contraception; NR = not reported (small cell size). * Adjusted for age group, concessional status, state/territory, and calendar year. |
|||||||||||||||
Box 6 – Frequency of contraceptive overlap at the time of each dispensing of category X medications dispensed more than once to individual women aged 15–49 years, Australia, 2013–2021, by Anatomical Therapeutic Chemical (ATC) level 1 category
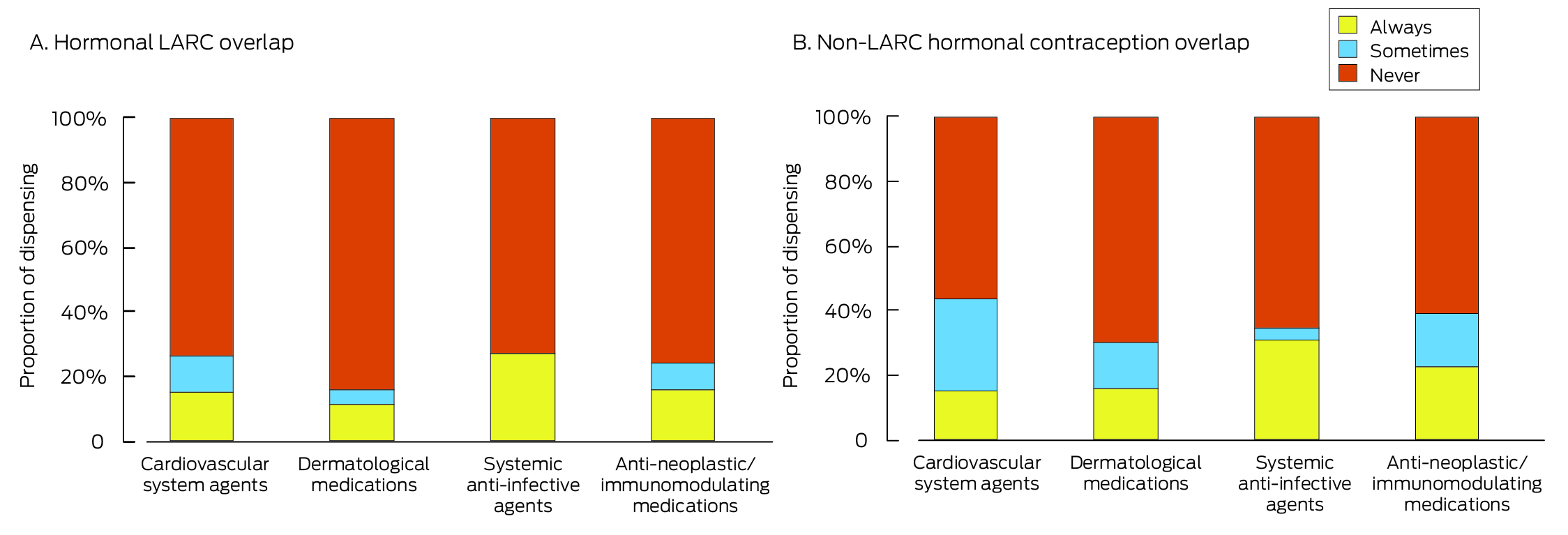
LARC = long‐acting reversible contraception. The data underlying these graphs are available in the Supporting Information, table 6.
Received 7 April 2023, accepted 11 December 2023
- Luke E Grzeskowiak1,2
- Vivienne Moore3
- Kelly Hall3
- Jenni Ilomäki4
- Danielle Schoenaker5
- Elizabeth Lovegrove5
- Danielle Mazza6
- Kirsten I Black7,8
- Debra S Kennedy9,10
- Michael J Davies11
- Alice Rumbold2,11
- 1 College of Medicine and Public Health, Flinders University, Adelaide, SA
- 2 South Australian Health and Medical Research Institute (SAHMRI), Adelaide, SA
- 3 The University of Adelaide, Adelaide, SA
- 4 Centre for Medicine Use and Safety, Monash University, Melbourne, VIC
- 5 University of Southampton, Southampton, United Kingdom
- 6 Monash University, Melbourne, VIC
- 7 The University of Sydney, Sydney, NSW
- 8 Royal Prince Alfred Hospital, Sydney, NSW
- 9 The Royal Hospital for Women, Sydney, NSW
- 10 University of New South Wales, Sydney, NSW
- 11 Robinson Research Institute, University of Adelaide, Adelaide, SA
Open access:
Open access publishing facilitated by Flinders University, as part of the Wiley – Flinders University agreement via the Council of Australian University Librarians.
Data sharing:
We have full access to all data (including statistical reports and tables) for this study. The data underlying this report cannot be publicly shared because of ethical or privacy reasons, but may be shared upon reasonable request to the corresponding author.
This study was supported by a Medical Research Future Fund Cardiovascular Health Mission: Congenital Heart Disease grant (ARGCHDG000041). Luke Grzeskowiak receives salary support from a Channel 7 Children's Research Foundation Fellowship (CRF‐210323).
No relevant disclosures.
- 1. McElhatton P. Principles of teratogenicity. Curr Obstet Gynaecol 1999; 9: 163‐169.
- 2. Therapeutic Goods Administration (Department of Health and Aged Care). Australian categorisation system for prescribing medicines in pregnancy. Update 4 May 2011. https://www.tga.gov.au/australian‐categorisation‐system‐prescribing‐medicines‐pregnancy (viewed Aug 2024).
- 3. Lammer EJ, Chen DT, Hoar RM, et al. Retinoic acid embryopathy. N Engl J Med 1985; 313: 837‐841.
- 4. Vargesson N. Thalidomide‐induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today 2015; 105: 140‐156.
- 5. Faculty of Sexual and Reproductive Health. Contraception for women using known teratogenic drugs or drugs with potential teratogenic effects (FSRH CEU statement). 1 Feb 2018. https://www.fsrh.org/Public/Public/Documents/fsrh‐ceu‐statement‐contraception‐for‐women‐using‐known‐teratogenic‐drugs.aspx (viewed Jan 2023).
- 6. Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: a review. JAMA 2021; 326: 2507‐2518.
- 7. Shroukh WA, Steinke DT, Willis SC. Risk management of teratogenic medicines: a systematic review. Birth Defects Res 2020; 112: 1755‐1786.
- 8. Pearson SA, Pesa N, Langton JM, et al. Studies using Australia's Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: a systematic review of the published literature (1987–2013). Pharmacoepidemiol Drug Saf 2015; 24: 447‐455.
- 9. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634.
- 10. Australian Bureau of Statistics. National, state and territory population, June 2022. 15 Dec 2022. https://www.abs.gov.au/statistics/people/population/national‐state‐and‐territory‐population/jun‐2022 (viewed Jan 2023).
- 11. Therapeutic Goods Administration. Database of adverse event notifications: medicines. https://apps.tga.gov.au/PROD/DAEN/daen‐entry.aspx (viewed Jan 2023).
- 12. Chan A, Keane RJ, Hanna M, Abbott M. Terminations of pregnancy for exposure to oral retinoids in South Australia, 1985–1993. Aust N Z J Obstet Gynaecol 1995; 35: 422‐426.
- 13. Kennedy D, Hill M, Cupitt D, et al. Isotretinoin: the Mothersafe experience 2010 to 2014. A need to revisit education and prescribing practices [abstract: 2nd International OTIS‐ENTIS conference at the Hospital for Sick Children, Toronto, Canada, 19–21 Sept 2014]. Birth Defects Res A Clin Mol Teratol 2014; 100: 519.
- 14. Raichand S, Pearson SA, Zoega H, et al. Utilisation of teratogenic medicines before and during pregnancy in Australian women. Aust N Z J Obstet Gynaecol 2020; 60: 218‐224.
- 15. Crijns HJMJ, van Rein N, Gispen‐de Wied CC, et al. Prescriptive contraceptive use among isotretinoin users in the Netherlands in comparison with non‐users: a drug utilisation study. Pharmacoepidemiol Drug Saf 2012; 21: 1060‐1066.
- 16. Ruiter R, Teichert M, Straus SMJM, et al. Concomitant use of contraceptives and potentially teratogenic medicinal products: results from a study using pharmacy dispensing data in the Netherlands. Reprod Sci 2012; 19: 987‐994.
- 17. Grzeskowiak LE, Calabretto H, Amos N, et al. Changes in use of hormonal long‐acting reversible contraceptive methods in Australia between 2006 and 2018: a population‐based study. Aust N Z J Obstet Gynaecol 2021; 61: 128‐134.
- 18. Mazza D, Bateson D, Frearson M, et al. Current barriers and potential strategies to increase the use of long‐acting reversible contraception (LARC) to reduce the rate of unintended pregnancies in Australia: an expert roundtable discussion. Aust N Z J Obstet Gynaecol 2017; 57: 206‐212.
- 19. Lortscher D, Admani S, Satur N, Eichenfeld LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol 2016; 15: 670‐674.
- 20. Celgene Corporation. Thalomid® (thalidomide) capsules (Australian product information). 6 Oct 2023. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP‐2010‐PI‐03581‐3&d=20240902172310101 (viewed Aug 2024).
- 21. Royal Australian College of General Practitioners. GP prescribing rights for isotretinoin [RACGP submission]. May 2014. Archived: https://webarchive.nla.gov.au/awa/20150226201654/http://aestheticmedicinecollege.org.au/pdf/racgpisotretinoinsubmission.pdf (viewed Jan 2023).
- 22. McElroy J. Maternity outcomes and access following regulatory changes for isotretinoin prescribing in New Zealand [Thesis, Master of Health Sciences]. University of Otago, 2017. http://hdl.handle.net/10523/7538 (viewed Sept 2023).
- 23. Mazza D, Watson CJ, Taft A, et al. Increasing long acting reversible contraceptives: the Australian Contraceptive ChOice pRoject (ACCORd) cluster randomized trial. Am J Obstet Gynecol 2020; 222: S921.e1‐S921.e13.
- 24. Skiba MA, Islam RM, Bell RJ, Davis SR. Hormonal contraceptive use in Australian women: who is using what? Aust N Z J Obstet Gynaecol 2019; 59: 717‐724.






Abstract
Objectives: To examine patterns in the dispensing of category X medications (Therapeutic Goods Administration categorisation system for prescribing medicines in pregnancy) to women aged 15–49 years in Australia during 2008–2021, and patterns of concurrent use of hormonal long‐acting reversible contraception (LARC) and other hormonal contraception.
Study design: Retrospective cohort study; analysis of 10% random sample of national Pharmaceutical Benefits Scheme dispensing data.
Participants, setting: Women aged 15–49 years dispensed category X medications, Australia, 1 January 2013 – 31 December 2021.
Main outcome measures: Incident and prevalent dispensing of category X medications, by medication class, age group, and year; contraceptive overlap (proportions of women dispensed hormonal LARC or other hormonal contraception that overlapped the first dispensing of category X medications), by medication class.
Results: Among 15 627 women aged 15–49 years dispensed category X medications during 2013–2021, the prevalence of dispensing increased from 4.6 in 2013 to 8.7 per 1000 women aged 15–49 years in 2021; the largest increase was for the dispensing of dermatological agents, from 3.9 to 7.9 per 1000 women aged 15–49 years. LARC overlap was inferred for 2059 women at the time of first dispensing of category X medications (13.2%); 3441 had been dispensed any type of hormonal contraception (22.1%). The proportion with LARC overlap was smallest for those dispensed dermatological agents (1806 of 14 331 women, 12.6%); for this drug class, both LARC overlap (adjusted odds ratio [aOR], 0.17; 95% confidence interval [CI], 0.14–0.20) and any hormonal contraception overlap (aOR, 0.28; 95% CI, 0.25–0.32) were less likely for those aged 15–19 years than for women aged 25–29 years.
Conclusions: Concurrent use of highly effective hormonal contraception at the time of first dispensing of category X medications is low in Australia, raising concerns about potential fetal harms during unintended pregnancies. Awareness of the importance of hormonal contraception and its uptake by women prescribed category X medications should be increased.