The early detection of adverse events following immunisation (AEFI) is essential to protect public health and to maintain confidence in vaccination. Vaccine pharmacovigilance — the monitoring, detection, investigation and actioning of vaccine safety signals — occurs across a collaborative landscape that includes the Therapeutic Goods Administration (TGA), the nationally funded surveillance initiative AusVaxSafety, and state and territory health departments.
The purpose of these pharmacovigilance systems is to monitor for unexpected types and rates of AEFI, including rare and population‐specific events.1,2 The identification and investigation of potential safety concerns is a key function of these systems, with the TGA also focused on ensuring that vaccines registered for use in Australia maintain a favourable benefit–risk profile.
The post‐market safety monitoring of vaccines supports Australia's seasonal influenza vaccination program. Annual influenza vaccination is recommended for all individuals over six months of age and is fully funded for at‐risk groups through the National Immunisation Program.3 The widespread distribution of seasonal influenza vaccines, particularly to children and at‐risk groups through the National Immunisation Program, and annual formulation changes to cover new virus strains mean vaccine pharmacovigilance systems need to be sensitive and responsive.
In 2023, about 9 million seasonal influenza vaccines were administered to Australians.4 This was substantially fewer than the 11 million influenza vaccines administered in 2022, with notably lower coverage in Aboriginal and Torres Strait Islander peoples.5,6 Although there are varied and complex reasons that affect vaccine uptake, this article aims to support the seasonal influenza vaccine program by providing confidence in the robust national pharmacovigilance processes for seasonal influenza vaccines in Australia.
The TGA and seasonal influenza vaccine pharmacovigilance
All AEFI reported to the TGA are used in the TGA's pharmacovigilance processes and contribute to the monitoring of a vaccine's safety profile. AEFI reports submitted to the TGA are entered into the TGA's internal national post‐market safety database — the Australian Adverse Event Management System (AEMS). This database receives spontaneous (passive) reports for medicines and vaccines submitted by health care professionals, pharmaceutical companies (Australian sponsors), state and territory health departments, and the public. Australian sponsors are required under legislation to report serious adverse reactions to the TGA.7
In most states and territories, health care providers have a statutory obligation to report certain AEFI through state and territory reporting channels. These reports are submitted to the TGA and are included in AEMS. The TGA also shares AEFI information with states and territories, in accordance with cooperative data‐sharing arrangements. The states and territories are key contributors to the national collection of AEFI, accounting for the majority of the AEMS seasonal influenza vaccine dataset in 2023.
All adverse event reports must include details of the suspected adverse event and product involved, patient information such as age or date of birth, as well as details of the reporter. The TGA may seek additional follow‐up information for cases with insufficient details, which is typically facilitated by the relevant state or territory health departments.
After the TGA receives a report, the reported adverse events are coded using the standardised Medical Dictionary for Regulatory Activities (MedDRA). Information from these reports is made publicly available through the TGA's Database of Adverse Event Notifications. Some reports may not be included if the report was found to be a duplicate or if the report was not valid; for example, the adverse event occurred before the vaccine was administered.
Adverse event reports may be classified as “serious” (as defined by the World Health Organization) if they involve the following outcomes:
- fatal or life‐threatening condition/s;
- new or prolonged hospitalisation;
- persistent or significant disability;
- congenital anomaly or birth defect; and
- any medical event that requires an intervention to prevent the above outcomes.8
The assessment of an AEFI as serious may be determined by either the reporter or during internal TGA review. The TGA identifies and reviews cases of regulatory significance, including serious AEFI reports of concern, and may consider convening the Vaccine Safety Investigation Group when an AEFI of concern is identified that has the potential to change the favourable benefit–risk balance of a vaccine, or affect public confidence in vaccine safety. This independent expert panel may undertake causality assessments to assist in the investigation of either individual AEFI or safety signals of concern. The outcomes from the Vaccine Safety Investigation Group are publicly available and serve to inform regulatory action and the vaccine program.
In addition to individual case review, the TGA also identifies potential influenza vaccine safety signals through data mining algorithms. This recognised method of signal detection is based on the concept of disproportionality and provides a measure of whether or not an adverse event associated with a drug (termed a drug‐adverse event pair) is reported more frequently compared with all other drug‐adverse event pairs in a background dataset.9,10 The TGA uses data‐mining algorithms to interrogate the AEMS database and to identify influenza vaccine‐adverse event pairs that have a greater number of reports than expected. Disproportionality analyses for seasonal influenza vaccines are performed monthly based on vaccine antigens and trade names (brands).
The TGA's vaccine pharmacovigilance activities are also informed by local data external to AEMS, and international data. The TGA may investigate potential signals based on interagency communication (including notification of potential signals from AusVaxSafety), meetings with state and territory health authorities (TGA AEFI–Jurisdictional Immunisation Coordinator meetings), international regulators, pharmaceutical companies, or from published literature2 (Box). The TGA's investigations consider individual AEFI reports, national reporting rates and observed versus expected case numbers (using dose data derived from the Australian Immunisation Register), global disproportionality findings in the World Health Organization Vigibase (a global database of adverse event case reports), published literature, and the specificity and biological plausibility of the adverse event. Advice on a safety signal may also be requested from the Advisory Committee on Vaccines to inform any regulatory action taken to reduce the risk of an adverse event.
Seasonal influenza vaccines are also monitored through monthly safety surveillance reports that are completed throughout the influenza season. These reports include review and documentation of the most frequently reported AEFI associated with seasonal influenza vaccines, and give a detailed review of adverse events of special interest including pyrexia, febrile convulsion, seizure, and Guillain–Barré syndrome. Adverse events of special interest associated with seasonal influenza vaccine brands are analysed, with consideration given to report numbers (weekly, monthly and cumulative), reporting rates, and proportional reporting ratio trend analyses. Observed versus expected analyses for Guillain–Barré syndrome are also conducted.
These monthly updates are shared with states and territories, the National Centre for Immunisation Research and Surveillance, and AusVaxSafety during regular TGA AEFI– Jurisdictional Immunisation Coordinator meetings. The Australian Technical Advisory Group on Immunisation is also provided with routine surveillance updates on seasonal influenza vaccines for consideration in the broader context of the National Immunisation Program.
New vaccines registered on the Australian Register of Therapeutic Goods or added to the National Immunisation Program, including the respiratory syncytial virus vaccines Arexvy (GSK) and ABRYSVO (Pfizer) as well as Shingrix (GSK), are closely monitored through similar enhanced safety surveillance processes.
AusVaxSafety and seasonal influenza vaccine pharmacovigilance
AusVaxSafety has performed national active surveillance of brand‐ and age‐specific seasonal influenza vaccine safety annually since 2014.11 As part of routine surveillance, safety surveys are sent via SMS or email to influenza vaccine recipients vaccinated at a representative sample of sentinel sites including general practices, pharmacies, Aboriginal Community Controlled Health Organisations, and state health vaccination centres. Surveys are sent to individuals three days after they received an influenza vaccine. Adverse event data are then analysed, and fast initial response cumulative summation (FIR CUSUM) safety signal detection is performed on a weekly basis for both fever (among children aged under five years) and medical attendance (including presentation to a general practice, Aboriginal health worker, and/or an emergency department). The FIR CUSUM signal detection method tracks the relative likelihood that the underlying event rate (fever or medical attendance) is at the maximum acceptable rate compared with the likelihood it is at the lower expected rate, given the accumulated data and pre‐specified values for the expected and maximum acceptable event rate parameters, and the signal threshold.12 The results and outcomes of these signal detection processes are reported to the Australian Government Department of Health and Aged Care, including the TGA, and state and territory jurisdictions. Summaries of adverse event data are also reported to the public on the AusVaxSafety website.13
In order to align methodologically with the coronavirus disease 2019 (COVID‐19) vaccine safety surveillance that commenced in March 2021, influenza vaccine safety surveillance was updated in 2022.14 The safety survey was updated with regard to the questions asked (to match adverse events solicited following COVID‐19 vaccination) and the survey delivery method (one survey link sent, in contrast to the former multistage survey process). This update enables the collection of more informative safety data and enables consideration of individual and concomitant vaccination.
In addition, the Bayesian posterior predictive analysis method was added to influenza vaccine signal detection analyses from 2022. This analysis estimates the predicted distribution of future events (fever or medical attendance) conditional on the accumulated data each week. This predicted distribution adjusts for the age, sex, jurisdiction, Indigenous status and chronic medical condition within the population responding to the survey. A signal is flagged if the actual observed number of fever or medical attendance events exceeds the 99th percentile of the predicted distribution. The two signal detection methods differ in their sensitivity to longer term cumulative departures from a fixed expected baseline event rate (FIR CUSUM) or to shorter term acute changes in the event rate (posterior predictive analysis). As such, these statistical methods are complementary and allow for a comparison between the FIR CUSUM method and posterior predictive analysis method if a signal is detected.
Challenges of national pharmacovigilance systems
Although a diverse range of information is considered across Australia's national pharmacovigilance systems, there remain challenges associated with influenza vaccine signal detection and investigation. The use of spontaneous AEFI data in the TGA's pharmacovigilance system is affected by the inherent limitations associated with individual AEFI reporting including under‐reporting and incomplete reports. Under‐reporting of AEFI is a well recognised limitation of spontaneous adverse event reporting systems, with reporting affected by an individual's knowledge of and access to reporting pathways, as well as the perceived importance of reporting.15,16 As noted in a 2013 article,15 other important factors that affect reporting are the time required to report, confidence in reporting cases of uncertain causality, and the reporting of single and/or non‐serious reports. Accurate and timely reporting of AEFI supports rapid signal detection, with both individual case review and data‐mining pharmacovigilance methods dependent upon this action.17 Therefore, the TGA encourages all patients and clinicians to report any suspected AEFI and is committed to enhancing the reporting system to facilitate easy access. The concurrent active surveillance processes of AusVaxSafety also work in synergy to help mitigate this limitation by prompting individuals who received a vaccine to report AEFI. However, the quality of information provided in these self‐reports and the presence of missing data may have an impact on safety signal detection and investigation. For passive reports, state and territory health departments support the TGA through attainment of additional detailed information on individual cases. Reporters are encouraged to provide as much information as possible either at the time of reporting an adverse event, or when further information is sought. The identification of long term or late‐onset reactions is another challenge for pharmacovigilance systems and is not captured in the day 3 influenza vaccine surveillance surveys. Their detection requires clinicians and individuals to be alert to the possible involvement of vaccines in chronic or late‐onset conditions, and to consider reporting these to the TGA. Despite recognised limitations, passive surveillance has a demonstrated ability to rapidly detect safety signals and has enabled prompt regulatory action including updates to the product information of seasonal influenza vaccines.
Future directions
Ongoing improvements to Australia's national pharmacovigilance systems are underway to enhance data capture and to ensure the continued safety of all medicines available on the Australian market, including seasonal influenza vaccines. Current work being explored by the TGA includes improvements in AEFI data quality and completeness including ethnicity and Aboriginal and Torres Strait Islander reporting, and disproportionality methodologies. Updates to the TGA's internal database also aim to increase the functionality of the system, including future opportunities for more streamlined data exchange. The TGA is actively involved in ensuring that planned enhancements align with the broader health interoperability work. Education modules are also being developed to increase health practitioner awareness and engagement with adverse event reporting.
The TGA and AusVaxSafety recognise the importance of providing timely and transparent information on seasonal influenza vaccine safety to the Australian public. Close collaboration between national pharmacovigilance systems continues to support the health and safety of Australians through close monitoring of seasonal influenza vaccines and rapid communication of any emerging safety signals.
Box – Overview of national seasonal influenza vaccine adverse events following immunisation surveillance in Australia in 2024
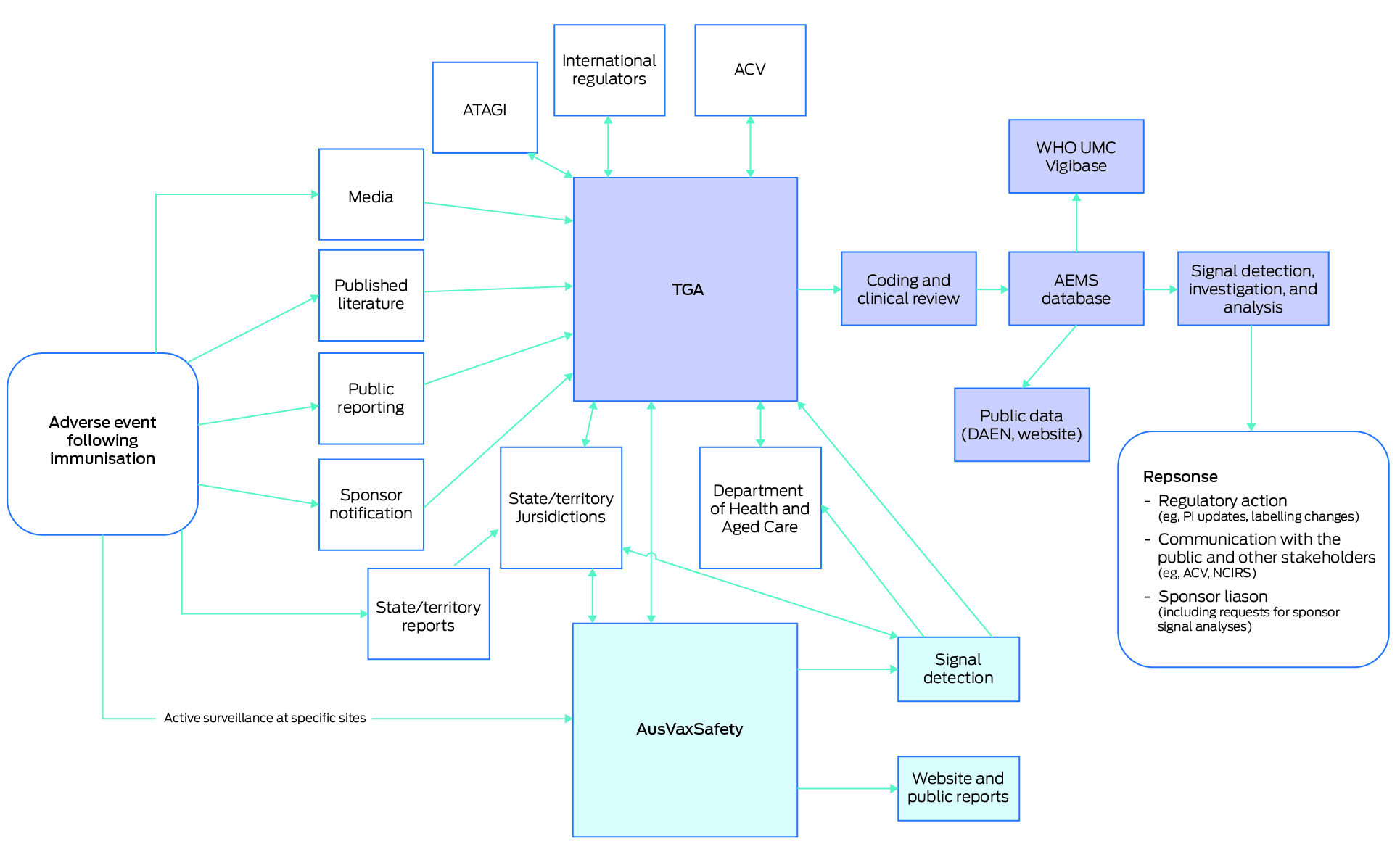
ACV = Advisory Committee on Vaccines; AEMS = Adverse Events Management System; ATAGI = Australian Technical Advisory Group on Immunisation; DAEN = Database of Adverse Event Notifications; NCIRS = National Centre for Immunisation Research and Surveillance; PI = product information; TGA = Therapeutic Goods Administration; UMC = Uppsala Monitoring Centre; WHO = World Health Organization.
Provenance: Not commissioned; externally peer reviewed.
- 1. AusVaxSafety. Safety data. Sydney: AusVaxSafety, 2024. https://ausvaxsafety.org.au/safety‐data (viewed Mar 2024).
- 2. Therapeutic Goods Administration, Department of Health and Aged Care. TGA safety monitoring of medicines. Canberra: Commonwealth of Australia, 2018. https://www.tga.gov.au/safety/safety/safety‐monitoring‐medicines/tga‐safety‐monitoring‐medicines (viewed Mar 2024).
- 3. Australian Technical Advisory Group on Immunisation (ATAGI), Department of Health and Aged Care. Statement on the administration of seasonal influenza vaccines in 2024. Canberra: Commonwealth of Australia, 2024. https://www.health.gov.au/sites/default/files/2024‐02/atagi‐statement‐on‐the‐administration‐of‐seasonal‐influenza‐vaccines‐in‐2024.pdf (viewed Mar 2024).
- 4. Department of Health and Aged Care. Influenza (flu) immunisation data — 1 March to 3 October — 2020–2023. Canberra: Commonwealth of Australia, 2023. https://www.health.gov.au/resources/publications/influenza‐flu‐immunisation‐data‐1‐march‐to‐3‐october‐2020‐2023?language=en (viewed Mar 2024).
- 5. National Centre for Immunisation Research and Surveillance. Influenza vaccination coverage data. Sydney: NCIRS, 2023. https://ncirs.org.au/influenza‐vaccination‐coverage‐data (viewed Mar 2024).
- 6. National Centre for Immunisation Research and Surveillance. Historical national influenza vaccination coverage, at end of year by age group and Aboriginal and Torres Strait Islander status, 2020–2022. Sydney: NCIRS, 2023. https://ncirs.org.au/influenza‐vaccination‐coverage‐data/historical‐national‐influenza‐vaccination‐coverage‐2020‐2022 (viewed Mar 2024).
- 7. Therapeutic Goods Administration, Department of Health and Aged Care. Pharmacovigilance responsibilities of medicine sponsors. Canberra: Commonwealth of Australia, 2023. https://www.tga.gov.au/resources/resource/guidance/pharmacovigilance‐responsibilities‐medicine‐sponsors (viewed Mar 2024).
- 8. World Health Organization. Global manual on surveillance of adverse events following immunisation, 2016 update. Geneva: WHO, 2016. https://www.who.int/publications/i/item/9789241507769 (viewed Mar 2024).
- 9. Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009; 18: 427‐436.
- 10. Hochberg AM, Hauben M, Pearson RK, et al. An evaluation of three signal‐detection algorithms using a highly inclusive reference event database. Drug Saf 2009; 32: 509‐525.
- 11. Pillsbury AJ, Cashman P, Leeb A, et al. Real‐time safety surveillance of seasonal influenza vaccines in children, Australia, 2015. Euro Surveill 2015; 20; https://doi.org/10.2807/1560‐7917.ES.2015.20.43.30050.
- 12. Pillsbury AJ, Glover C, Jacoby P, et al. Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia. BMJ Open 2018; 8: e023263.
- 13. AusVaxSafety. Influenza vaccines. Sydney: AusVaxSafety, 2022. https://ausvaxsafety.org.au/safety‐data/influenza‐vaccine (viewed Mar 2024).
- 14. Deng L, Glover C, Dymock M, et al. The short term safety of COVID‐19 vaccines in Australia: AusVaxSafety active surveillance, February–August 2021. Med J Aust 2022; 217: 195‐202. https://www.mja.com.au/journal/2022/217/4/short‐term‐safety‐covid‐19‐vaccines‐australia‐ausvaxsafety‐active‐surveillance#:~:text=Adverse%20events%200%E2%80%933%20days,reported%20symptoms%20
- 15. Palleria C, Leporini C, Chimirri S, et al. Limitations and obstacles of the spontaneous adverse drugs reactions reporting: two “challenging” case reports. J Pharmacol Pharmacother 2013; 4: S66‐S72.
- 16. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015; 33: 4398‐4405.
- 17. Hauben M, Horn S, Reich L. Potential use of data‐mining algorithms for the detection of “surprise” adverse drug reactions. Drug Saf 2007; 30: 143‐155.






AusVaxSafety surveillance is funded under a contract with the Australian Department of Health and Aged Care. The authors acknowledge the participants and staff at the surveillance sites, state and territory health departments, and Telethon Kids Institute, and the contribution of the surveillance tools SmartVax, Vaxtracker, and Microsoft COVID Vaccine Management System. The authors also wish to thank the Therapeutic Goods Administration staff of the Vaccines Surveillance Section, Adverse Event and Medicine Defects Section, and Technical and Safety Improvement Section, who support the safety surveillance of influenza vaccines.
No relevant disclosures.