Clinical record
In August 2023, an otherwise healthy 54‐year‐old woman presented to hospital with acute onset fevers and sweats, followed by four days of nausea, vomiting, generalised severe abdominal pain and diarrhoea. The diarrhoea was non‐bloody, with more than ten bowel motions per day. The patient worked as a support worker for older people, and denied previous diarrhoeal illness, sick contacts, consumption of undercooked food, and recent travel.
On presentation, she was hypotensive (blood pressure, 72/45 mmHg), tachycardic (105 beats/minute) and febrile (38.9°C). Respiratory rate and oxygen saturations were normal. Her abdomen was globally tender, maximal in the right lower quadrant, without peritonism. No rash was evident.
Initial investigations revealed an acute kidney injury (serum creatinine, 261 μmol/L; reference interval [RI], 45–90 μmol/L; baseline unknown but reportedly normal); mild transaminitis (alanine transaminase, 67 U/L [RI, 10–35U/L]; aspartate aminotransferase, 51 U/L [RI, 10–35 U/L]); neutrophilic leucocytosis (neutrophil count, 16.9 × 109/L [RI, 2–7 × 109/L]); and an elevated C‐reactive protein level of 423.4 mg/L (RI, ≤ 4.9 mg/L). The patient's lactate level was normal (1.9 mmol/L [RI ≤ 1.9mmol/L]). Computed tomography (abdomen and pelvis) showed severe pancolitis, with diffuse mural thickening from the ascending colon to the rectum, and trace free fluid in the pelvis (Box).
Empirical intravenous ceftriaxone and metronidazole were commenced, with clindamycin added due to concern for toxic shock syndrome, despite a lack of mucocutaneous features.1 Despite 4 L of crystalloid resuscitation, the patient required intensive care unit admission for vasopressors.
Blood cultures isolated gram‐positive cocci in chains within 12 hours, subsequently identified as Streptococcus pyogenes with clonal type ST982 (emm67 type) by whole‐genome sequencing (Illumina) analysis. A screen for virulence factors revealed DNases (MF/Spd), streptolysin O, and group A Streptococcus (GAS) superantigens (speB, speA, speG, speH and speK).
No gastrointestinal pathogens were identified. Faecal nucleic acid amplification was negative for viral, bacterial and parasitic targets using commercially validated multiplexed panels: Faecal Pathogens M 16‐well (Ausdiagnostics) and Biofire FilmArray Gastrointestinal Panel (bioMérieux). A panel of enteric bacterial cultures was negative, including cultures on xylose–lysine–deoxycholate, thiosulfate–citrate–bile salts and cefsulodin–irgasan–novobicin agars. A colonoscopy on day 7 showed macroscopically normal large bowel mucosa. Histopathology of random biopsies of the colon and terminal ileum were unremarkable.
Vasopressors were weaned from day 2 and treatment was rationalised to benzylpenicillin monotherapy. The patient defervesced and had normal bowel motions by day 5, and transitioned to oral amoxicillin to complete ten days of therapy. Household contacts were counselled about the risk of disease but remained well. By the time of follow‐up a fortnight after completing antimicrobial therapy, the patient remained well and did not develop any further symptoms, including no convalescent rash or desquamation.
Discussion
Invasive group A streptococcal (iGAS) disease is caused by infection of sterile sites by S. pyogenes (GAS), an aerobic, β‐haemolytic, gram‐positive coccus. Asymptomatic skin or pharyngeal carriage is present in 5–30% of the community, and person‐to‐person transmission occurs by inhalation of respiratory droplets or direct skin contact.2
In Australia, iGAS disease disproportionately affects Indigenous communities and regional and remote communities, with some of the highest prevalence rates worldwide found in Central Australia.3 In mid‐2021, iGAS disease became notifiable to the National Notifiable Diseases Surveillance System.4
GAS infections have variable clinical presentations. Most common are pharyngitis, with or without scarlet fever, and uncomplicated skin and soft tissue infections, including cellulitis and impetigo. Severe, acute complications include streptococcal toxic shock syndrome (STSS) and iGAS disease, which includes bacteraemia, septic arthritis, osteomyelitis, necrotising fasciitis, and puerperal sepsis. Delayed‐onset, non‐suppurative complications include acute rheumatic fever and post‐streptococcal glomerulonephritis.2
Our patient had a rare manifestation of iGAS disease with severe pancolitis and refractory shock. Common infective causes had been excluded. Additionally, ischaemic colitis was unlikely given the normal venous lactate level and radiological findings of pancolitis. Inflammatory bowel disease was excluded on colonoscopy findings and histopathology. Although diarrhoea may feature in STSS, our patient lacked the severe mucocutaneous findings of STSS. It is likely that the colitis was primarily toxin‐mediated given the normal macroscopic and microscopic findings.
Gastrointestinal iGAS disease is poorly described outside of STSS and rare paediatric reports of perianal disease or proctitis. Limited case reports of haemorrhagic and non‐haemorrhagic colitis have been described in children and young adults.5,6,7 Other virulent organisms, such as S. pneumoniae, can rarely present with abdominal symptoms or colitis.8
There is a seasonal global surge of iGAS disease, primarily caused by variants of the emm1 type, which has been responsible for severe iGAS disease presentations to hospital.9 By contrast, emm67 is a less commonly isolated emm type of S. pyogenes, which has poorly described tissue tropism and virulence. It has been identified in geographically disparate countries, and has been associated predominantly with pharyngitis, STSS‐associated speA gene expression, and in vitro tetracycline resistance.10,11 There are no previous reports of colitis caused by emm67.
This is an unusual adult case of iGAS disease presenting predominantly with symptoms of colitis without severe mucocutaneous manifestations. Rare presentations of acute streptococcal infection should be considered in the differential diagnosis for colitis with marked systemic features.
Lessons from practice
- There is a seasonal global surge of invasive group A streptococcal (iGAS) disease, particularly caused by Streptococcus variants of the emm1 type. Remarkably, the surge in Australia began out of season and continues to cause severe presentations in both adult and paediatric patients.
- Gastrointestinal manifestations of iGAS disease are uncommon outside the syndrome of streptococcal toxic shock syndrome in adults and paediatric cases of proctitis or perianal disease. iGAS disease should be considered in the differential diagnosis for patients presenting with sepsis.
- Household contacts should be counselled to seek medical attention for symptoms of potential iGAS disease within the 30 days of exposure. Medical prophylaxis of contacts may be considered.
Box – Computed tomography of the abdomen showing pancolitis
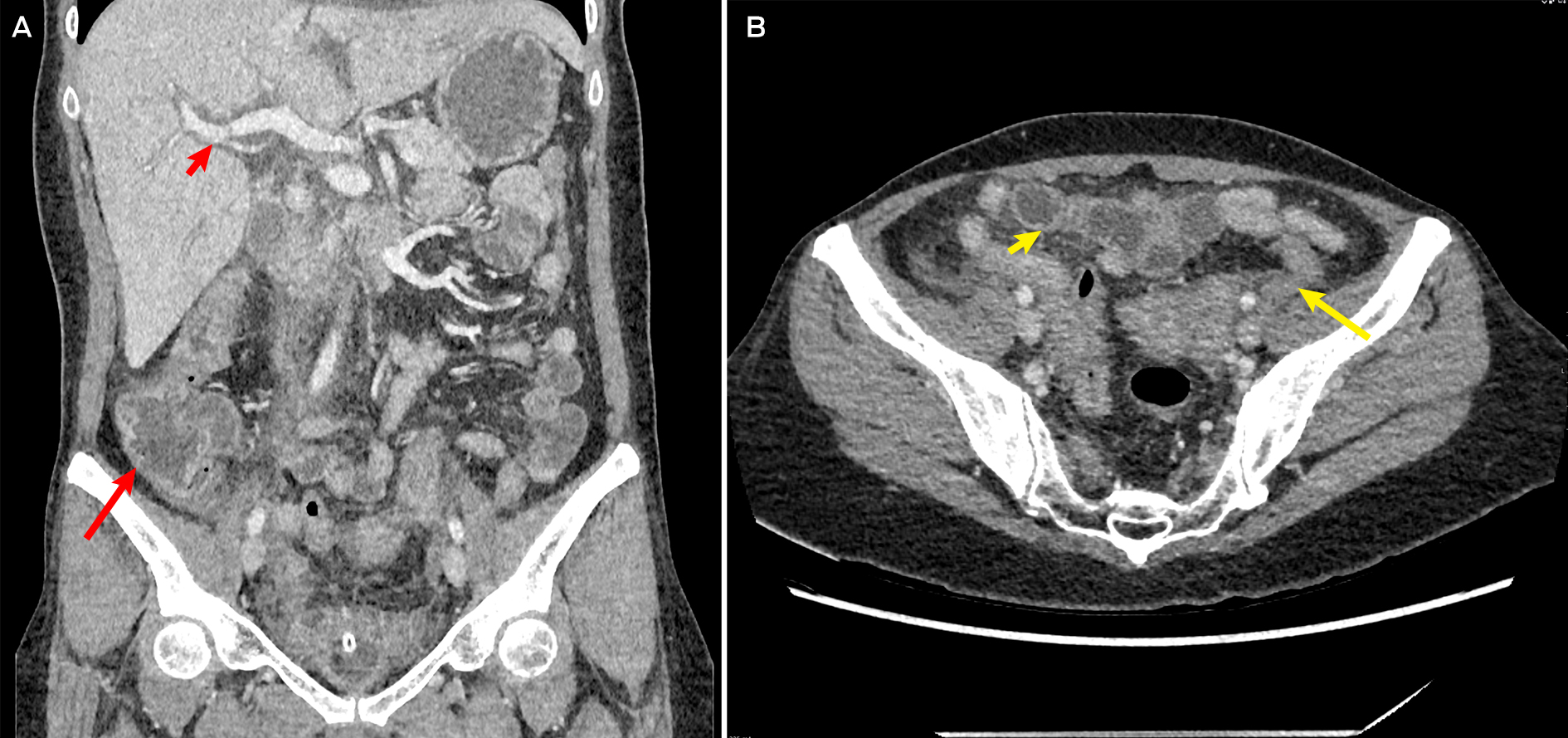
A: Representative coronal slice showing prominent caecal and ascending colon mural thickening (long arrow) and periportal thickening (short arrow), suggestive of pancolitis. B: Axial slice showing prominent sigmoid (long arrow) and transverse colon (short arrow) mural thickening, consistent with overall diagnosis of pancolitis.
Provenance: Not commissioned; externally peer reviewed.
- 1. Breiman RF, Davis JP, Facklam RR, et al. Defining the group a streptococcal toxic shock syndrome, rationale and consensus definition. JAMA 1993; 269: 390‐391.
- 2. Brouwer S, Rivera‐Hernandez T, Curren BF, et al. Pathogenesis, epidemiology and control of group A Streptococcus infection. Nat Rev Microbiol 2023; 21: 431‐447.
- 3. Birrel JM, Boyd R, Currie BJ, et al. Socio‐environmental and clinical features of invasive group A streptococcal disease in the Northern Territory of Australia. Commun Dis Intell (2018) 2023; 47; https://doi.org/10.33321/cdi.2023.47.39.
- 4. Department of Health and Aged Care. Group A streptococcal disease – invasive (iGAS). https://www.health.gov.au/diseases/group‐a‐streptococcal‐disease‐invasive‐igas (viewed Aug 2023).
- 5. Maraj B, Huang A, Patel S. Acute colitis in a patient with Streptococcus pyogenes bacteraemia. Am J Med 2018; 131: e13‐e14.
- 6. Isozaki A, Matsubara K, Yui T, et al. Group A B‐haemolytic streptococcal hemorrhagic colitis complicated with pharyngitis and impetigo. J Infect Chemother 2007; 13: 411‐413.
- 7. Arthur C, Linam LE, Linam WM. Group A beta‐haemolytic streptococcal colitis with secondary bacteraemia. Pediatr Infect Dis J 2012; 31: 1093‐1095.
- 8. Ronnachit A, Ellenberger KA, Gray TJ, et al. Streptococcus pneumoniae causing intra‐abdominal and pelvic infection: a case series. Cureus 2017; 9: e1967.
- 9. Davis MR, Keller N, Brouwer S, et al. Detection of Streptococcus pyogenes M1UK in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat Commun 2023; 14: 1051.
- 10. Tewodros W & Kronvall G. M Protein gene (emm type) analysis of group A beta‐hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol 2005; 43: 4369‐4376.
- 11. Sakota V, Fry A, Lietman T, et al. Genetically diverse group A streptococci from children in far‐western Nepal share high genetic relatedness with isolates from other countries. J Clin Microbiol 2006; 44: 2160‐2166.





Patient consent:
The patient provided written consent for publication.
We thank the Microbial Genomics Laboratory, NSW Health Pathology – Institute of Clinical Pathology and Medical Research, for genomic analysis of the isolate.
No relevant disclosures.