Clinical record
A 76‐year‐old female patient was urgently referred to the endocrine clinic for suppressed morning serum cortisol levels (< 28 nmol/L; reference range [RR], 138–650 nmol/L). She reported fatigue, limb bruising, hair thinning, mood irritability and 3–4 kg weight loss over the past year.
Her medical history included oral lichen planus diagnosed two years prior, with symptoms of dry mouth and discomfort, along with osteopenia and facial dermatitis. Regular medications included vaginal oestrogen, calcium carbonate, vitamin D and vitamin B complex. She denied glucocorticoid or opioid use.
The patient disclosed the continuous use of “magic mouthwash”, a compounded mouthwash prescribed by her oral medicine specialist for the past 12 months, with clinical improvement. Ingredients from the prescription label (Box 1) included clobetasol propionate, which is a potent topical corticosteroid. The mouthwash was used as directed without accidental ingestion.
Examination revealed signs of Cushing syndrome, including facial swelling, skin thinning, proximal muscle weakness, and bruising. Her oral cavity showed mild erythema and reticular white striae involving the buccal mucosae and ventral tongue, with no ulcerations or erosions.
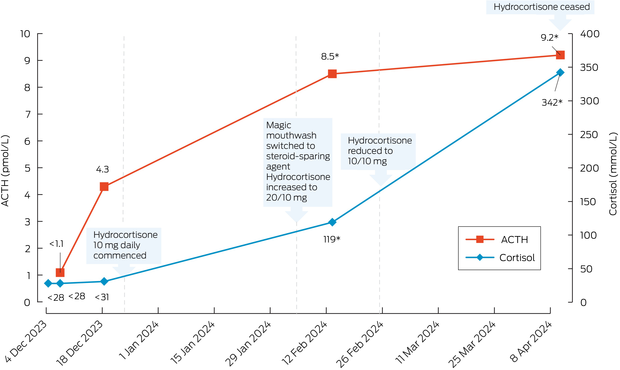
Laboratory tests confirmed secondary adrenal insufficiency with cortisol below 28 nmol/L and adrenocorticotropic hormone (ACTH) below 1.1 pmol/L (RR, <12.1 pmol/L). Other pituitary hormones were normal. Short synacthen test (SST) indicated inadequate response with peak cortisol 125 nmol/L at 60 minutes (RR, >420 nmol/L at the Royal Prince Alfred Hospital).
The patient was commenced on oral hydrocortisone 10 mg daily and referred to immunology for consideration of a steroid‐sparing agent. After transitioning from the magic mouthwash to the steroid‐sparing agent, the hydrocortisone dosage was increased to 20 mg in the morning and 10 mg in the early afternoon. The patient's symptoms improved, and hydrocortisone was tapered to 10 mg twice daily three weeks later.
A follow‐up evaluation two months after stopping the magic mouthwash demonstrated recovery of the hypothalamic–pituitary–adrenal (HPA) axis, with morning cortisol 342 nmol/L and paired ACTH 9.2 pmol/L. The patient was advised to stop the hydrocortisone 10 mg twice daily, and an SST performed ten days later confirmed an excellent response with peak cortisol 599 nmol/L at 60 minutes. Oral lichen planus remained well managed throughout. Biochemistry trends are shown in Box 2.
Discussion
This case underscores the need for vigilance in recognising the potential systemic absorption and adrenal suppressive effects of topical corticosteroids, even when used as part of dental treatments. Absorption was likely due to previous mucosal damage that may have healed with ongoing mouthwash use.1
Magic mouthwash is a prescription mouthwash that has gained popularity for the treatment of recurrent aphthous ulcers and oral mucositis related to autoimmune diseases (such as oral lichen planus and Behçet disease) and cancer therapies (such as chemotherapy and radiotherapy).2,3,4 These mixtures are compounded differently by individual pharmacies, with no fixed formulations. There are few randomised control trial data supporting their use.2,5,6 Typically, medications from at least three of the following classes are used: corticosteroid, antibiotic, antifungal, anaesthetic, antihistamine and antacid. Common corticosteroids include clobetasol propionate, dexamethasone, betamethasone and hydrocortisone. The solution is swished and spit out to prevent systemic side effects such as nausea and drowsiness. Usage duration guidelines are not standardised.
Long term use of glucocorticoids can lead to iatrogenic Cushing syndrome and secondary adrenal insufficiency and have been observed with topical preparations including gels and mouthwashes.1,7,8 A study involving 62 patients with oral lichen planus found that treatment with 0.05% clobetasol propionate mouthwashes for five minutes three times daily (initial phase) resulted in HPA axis suppression in 85.5% of patients.7 When the regimen was reduced to one mouthwash every other day (maintenance phase), HPA axis suppression decreased significantly to 4%, indicating a transient suppression effect. Cushingoid features, observed in 8% of patients, resolved upon dose reduction of the clobetasol propionate. The minimum concentration of topical clobetasol propionate required to induce iatrogenic Cushing syndrome and secondary adrenal insufficiency remains unknown.1
In affected cases, oral hydrocortisone therapy is recommended after stopping steroid mouthwash to prevent life‐threatening adrenal crisis. Although specific conversion rates between topical clobetasol propionate and oral hydrocortisone are unavailable, a daily dose of 10–30 mg of hydrocortisone is recommended for managing secondary adrenal insufficiency.9 The Endocrine Society guidelines suggest gradual tapering of hydrocortisone with close monitoring for signs and symptoms of adrenal insufficiency. HPA axis recovery is likely if the morning cortisol is greater than 300 nmol/L.10
In summary, we hope this case will raise awareness of the potential impact of magic mouthwash. HPA axis testing and an endocrine review are strongly advised for patients who exhibit cushingoid features while using steroid mouthwash, or who experience symptoms suggestive of adrenal insufficiency after discontinuation.
Lessons from practice
- Magic mouthwash is increasingly prescribed for the treatment of recurrent aphthous ulcers and oral mucositis related to autoimmune diseases or cancer treatments. It contains various ingredients, which may include high potency corticosteroids.
- The systemic absorption of corticosteroids from mouthwashes is a risk, especially if erosive oral lesions are present. This can lead to iatrogenic Cushing syndrome and secondary adrenal insufficiency.
- Hypothalamic–pituitary–adrenal axis testing and endocrine review is recommended for patients who exhibit cushingoid features while using steroid mouthwash or who show symptoms of adrenal insufficiency upon discontinuation.
Provenance: Not commissioned; externally peer reviewed.
- 1. Varoni EM, Molteni A, Sardella A, et al. Pharmacokinetics study about topical clobetasol on oral mucosa. J Oral Pathol Med 2012; 41: 255‐260.
- 2. McElhiney LF. Magic mouthwashes — a literature review and discussion of common compositions. Int J Pharm Compd 2024; 28: 94‐98.
- 3. Thongprasom K, Prapinjumrune C, Carrozzo M. Novel therapies for oral lichen planus. J Oral Pathol Med 2013; 42: 721‐727.
- 4. Sio TT, Le‐Rademacher JG, Leenstra JL, et al. Effect of doxepin mouthwash or diphenhydramine‐lidocaine‐antacid mouthwash vs placebo on radiotherapy‐related oral mucositis pain: the alliance A221304 randomized clinical trial. JAMA 2019; 321: 1481‐1490.
- 5. Uberoi AS, Brown TJ, Gupta A. Magic mouthwash for oral mucositis: a teachable moment. JAMA Intern Med 2019; 179: 104‐105.
- 6. Kravitz ND, Crutchfield WE, Miller S, et al. Magic mouthwash demystified. J Clin Orthod 2020; 54: 462‐465.
- 7. Gonzalez‐Moles MA, Scully C. HPA — suppressive effects of aqueous clobetasol propionate in the treatment of patients with oral lichen planus. J Eur Acad Dermatol Venereol 2010; 24: 1055‐1059.
- 8. Decani S, Federighi V, Baruzzi E, et al. Iatrogenic Cushing's syndrome and topical steroid therapy: case series and review of the literature. J Dermatolog Treat 2014; 25: 495‐500.
- 9. Gruber LM, Bancos I. Secondary adrenal insufficiency: recent updates and new directions for diagnosis and management. Endocr Pract 2022; 28: 110‐117.
- 10. Beuschlein F, Else T, Bancos I, et al. European Society of Endocrinology and Endocrine Society joint clinical guideline: diagnosis and therapy of glucocorticoid‐induced adrenal insufficiency. Eur J Endocrinol 2024; 190: G25‐G51.





Patient consent:
The patient gave written consent for publication.
No relevant disclosures.