Clinical record
A nine‐year‐old female patient presented with a two‐week history of vomiting, diarrhoea, abdominal pain, and a one‐week history of dysuria with macroscopic haematuria. There was no recent history of pharyngitis or a skin infection. Her past medical history included medication for attention deficit hyperactivity disorder, but no other significant conditions. On examination in the emergency department, the patient was tachycardic with heart rate 155 beats/minute (reference interval [RI], 70–130 beats/minute), febrile with temperature 38.8°C (RI, 36.4–37.4°C) and her abdomen was soft with generalised tenderness. There was no hypertension or evidence of oedema. Blood results showed leucocytosis with a white cell count of 15.7 × 109/L (RI, 4.5–13.5 × 109/L) and C‐reactive protein level of 110 mg/L (RI, < 3 mg/L). There was no associated evidence of toxic shock syndrome. Bedside urine analysis before antibiotic therapy showed leucocytes, blood and protein. The urine sample, as well as a blood and faecal samples were sent for microscopy and culture. Ultrasound scan of the kidneys, ureters and urinary bladder was unremarkable. The patient was admitted for presumed cystitis given little clinical evidence of glomerular nephritis and started on 750 mg intravenous cefazolin.
Overnight, the patient developed worsening abdominal pain, further vomiting and had ongoing fever. Abdominal examination revealed generalised peritonitis.
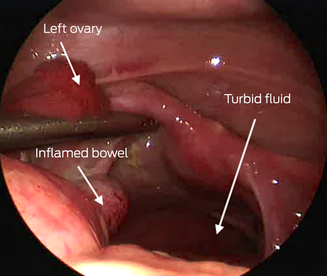
An abdominal ultrasound scan was reported as “suggestive for acute appendicitis”. The patient underwent diagnostic laparoscopy and appendicectomy. Intra‐operatively, the appendix appeared normal; however, throughout the abdomen there was turbid fluid and multiple fibrin deposits. The fallopian tubes and ovaries were erythematous (Box) but an intra‐operative gynaecological review yielded no concern for pelvic inflammatory disease. In/out catheterisation pre‐operatively drained urine with sediment, pus and blood and was again sent for microscopy and culture.
Both pre‐ and post‐antibiotic therapy urine microscopy and culture showed no significant bacterial growth. The patient had received two doses of 750 mg cefazolin intravenously before operative management. Post‐operatively, antimicrobial therapy was escalated to intravenous 4 g piperacillin/0.5 g tazobactam every 8 hours. The post‐operative course was uneventful; the patient received five days of intravenous antibiotics and was discharged on Day 5 with no further antimicrobial treatment.
The patient was well when reviewed two weeks after discharge. On review, the surgical histopathology reported “peri‐appendicitis with normal mucosa, moderate acute serositis and underlying neutrophil infiltrate involving the adventitia and muscularis propria only mildly”. Polymerase chain reaction and 16‐S molecular testing was not completed but would have been useful for the diagnosis. However, serum anti‐DNase B and antistreptolysin O titres were elevated, supporting the diagnosis of group A Streptococcus (GAS) primary peritonitis.
Discussion
In the mid‐20th century, primary peritonitis had an incidence of 10% of all abdominal emergencies with a mortality rate of 40–50%.1 Today there is a reported incidence of less than 2%.2,3 Interestingly, the global incidence of GAS infection has been increasing.4,5 In 2023, New South Wales Health distributed alerts to notify health practitioners and the community of the rising number of GAS infections and the subsequent increase in invasive group A Streptococcus infections (iGAS) presenting to our hospitals.6
The most common causative pathogens for primary peritonitis are Streptococcus pneumoniae, Staphyloccocus species and gram‐negative organisms; however, other causative agents have been reported.7,8 The pathogenesis is thought to be via haematogenous spread from the respiratory tract, skin or genitourinary tract.2,9,10 In this case, the likely origin was the urinary tract.
Conflicting symptoms and signs on presentation, in the absence of radiological findings, present a diagnostic challenge. Laparoscopy with peritoneal washout accompanied by appropriate antimicrobial therapy remains the recommended management of primary peritonitis.7,8,10 Appendicectomy is often performed to rule out intra‐abdominal causes of peritonitis (secondary peritonitis).
To our knowledge, this is the first Australian paediatric case (six‐month to 16‐year age group) of GAS primary peritonitis reported in the literature since 1994.10 The increased incidence of GAS infections may increase the incidence of GAS primary peritonitis.
Primary peritonitis is a rare disease that should remain a differential diagnosis in previously healthy children presenting with acute abdominal pain. This report aims to remind clinicians of this forgotten diagnosis, particularly in the context of an increasing incidence of GAS infections.
Lessons from practice
- The incidence of group A Streptococcus infections is increasing globally.
- Timely recognition and management of group A Streptococcus infections may decrease risk of the disease escalating to invasive group A Streptococcus infection.
- Primary peritonitis should remain a differential diagnosis for healthy children who present with acute abdominal pain.
- Operative management in conjunction with antimicrobial therapy remains the recommended treatment.
Provenance: Not commissioned; externally peer reviewed.
- 1. Fowler R. Primary peritonitis: changing aspects 1956‐1970. Aust Paediatr J 1971; 7: 73‐83.
- 2. Khilji MF. Primary peritonitis ‐ a forgotten entity. European J Pediatr Surg Rep 2015; 3: 27‐29.
- 3. Johnson CC, Baldessarre J, Levison ME. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis 1997; 24: 1035‐1045.
- 4. Westwood DA, Roberts RH. Management of primary group A streptococcal peritonitis: a systematic review. Surg Infect (Larchmt) 2013; 14: 171‐176.
- 5. Tapiainen T, Launonen S, Renko M, et al. Invasive group A streptococcal infections in children: a nationwide survey in Finland. Pediatr Infect Dis J 2016; 35: 123‐128.
- 6. New South Wales Department of Health. Community urged to be aware of rare serious bacterial illnesses requiring urgent care [media release]. 6 January 2023. https://www.health.nsw.gov.au/news/Pages/20230106_00.aspx (viewed Oct 2024).
- 7. Mayer MP, Schweizer P. Primary peritonitis in a child caused by Haemophilus parainfluenzae. Pediatr Surg Int 2002; 18: 728‐729.
- 8. Taylor A, Elliott BM, Atkinson J, et al. Group A Streptococcus primary peritonitis in children, New Zealand. Emerg Infect Dis 2023; 29: 2203‐2209.
- 9. Holden R, Wilmer A, Kollman T. Primary peritonitis due to group A Streptococcus in a previously healthy pediatric patient. Can J Infect Dis Med Microbiol 2012; 23: e69‐70.
- 10. Kimber CP, Hutson JM. Primary peritonitis in children. Aust N Z J Surg 1996; 66: 169‐170.





Patient consent:
The patient's parents provided written consent for publication.
No relevant disclosures.