Each year, approximately 1000 children in Australia and New Zealand, aged 0–14 years, are diagnosed with cancer. Despite paediatric cancer accounting for less than 1% of all cancer cases, the impact on their families and communities is profound and disproportionate.1,2,3 Paediatric brain cancers are the most significant cause of cancer‐related deaths within this age group, responsible for 40% of fatalities despite representing only 14% of diagnoses.2 Although significant advances in paediatric cancer treatments have pushed overall cure rates above 80%, the outlook for many brain tumour types remains bleak.4 Moreover, survivors often face lifelong clinical sequelae that severely diminish their quality of life,5 with 60% of survivors unable to reach independence in adulthood.6 This stark reality underscores the need for the expansion of clinical trials and integrated preclinical research aimed at improving outcomes for these individuals.
Barriers to clinical trials and the need for infrastructure funding
Conducting clinical trials in paediatric oncology faces many challenges, notably in regions with small populations spread across large geographic areas. Additional challenges include the rarity of each diagnosis, requiring international collaboration for patient accrual within feasible timelines.7,8,9 The limited duration and availability of grant funding complicates this, as the time for patient accrual and the generation of meaningful outcome data often surpasses funding periods. The specificity of diagnoses and the need for complex, multi‐agent therapies reduce the appeal of these trials to pharmaceutical companies, which favour single‐drug therapies. This necessitates consistent funding to maintain a skilled workforce in clinical trial conduct and monitoring. In this context, the Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) and the Australian Brain Cancer Mission (ABCM)10 have been addressing these challenges. By strategically deploying funds and fostering collaborations, ABCM and ANZCHOG have tackled the barriers that hinder clinical trial execution. ABCM provides the financial support needed for partnerships and trial capacity enhancement. ANZCHOG leads in navigating the challenges of paediatric oncology trials, building a network that overcomes logistical and financial constraints to facilitate progress and discovery.
The vision and role of Australian Brain Cancer Mission and the Central Nervous System Tumour Group
Initiated in 2017 with the targeted mission of enhancing brain cancer outcomes, the ABCM strategically mobilises resources, collaborations and research with the goal to double brain cancer survival rates.10 This mission is supported by a detailed strategic framework, blending substantial financial backing with extensive stakeholder engagement and a co‐funded model that unites government, philanthropic and private sectors. This strategy not only amplifies research funding but also fosters collaborative efforts across disciplines and borders, positioning it as a pioneering model for supporting rare cancers (Box 1).11 Complementing this aim, the ANZCHOG Central Nervous System (CNS) Tumour Group was established to lead the prioritisation of research areas, creating a clinical trial development pipeline that encompasses the stages of identification, development and funding application for trials (Box 2). This vision aims to unlock new therapeutic avenues.12,13,14,15,16 By harnessing a multidisciplinary approach and leveraging the ABCM, ANZCHOG's goal is to overcome the persistent challenges facing paediatric brain cancer treatment and research.
Achievements through collaboration and funding
Limited funding for paediatric brain tumour research, alongside challenges in complex trial execution for rare diseases, has hindered progress in paediatric brain cancer. The ABCM's introduction of a focused funding model, that prioritises international collaboration, combined with ANZCHOG's strategic planning, marked a shift towards a unified strategy. The collaboration between ABCM and ANZCHOG CNS Tumour Group has led to the initiation of or expansion to additional sites of 11 clinical trials (Box 3). These trials, addressing various tumour types including newly diagnosed and recurrent cases, signify a move forward in the field, demonstrating the impact of collaborative efforts to surpass traditional research barriers.17
The scope of these trials is broad, ranging from testing innovative treatments to improve cognitive and neurological benefits, as well as the quality of life of survivors. Each trial serves not only as a key source of insights into the disease mechanisms but also as a fundamental opportunity for direct patient benefit, potentially paving the way for the discovery of new therapeutic targets. An example of ABCM's impact is the conduct of trials such as SJ‐ELiOT, which leveraged the mission's funding to translate Australian research through international collaboration into potential new treatments.17 Additionally, the establishment of trials such as COZMOS (NCT03206021) and INFORM2 NivEnt (NCT03838042), funded through the ABCM, has been pivotal. COZMOS evaluated new combinational treatment protocols designed to improve survival rates for children with aggressive brain cancers, while INFORM2 NivEnt pioneers a precision medicine approach, tailoring treatments based on the genetic characteristics of individual tumours.
ANZCHOG's involvement in international consortia has significantly expanded, from our existing collaborations with international cooperative groups such as the Children's Oncology Group (COG)8 and the International Society of Paediatric Oncology (SIOP),9 with an additional six new partnerships (Box 3). This has allowed Australian and New Zealand children access to an increased number of global clinical trials, especially innovative early phase trials. This international dimension ensures that Australasian paediatric oncology remains at the forefront of global research efforts, benefiting from and contributing to worldwide advances in the field.
This portfolio of trials is a testament to the strategic planning of the ANZCHOG CNS Tumour Group and the ABCM. It underscores the critical importance of infrastructure funding in enabling the execution of complex clinical trials, and highlights the essential role of international collaboration in enhancing the scope and impact of research efforts. Through these collaborative initiatives, the barriers to clinical trial participation have been significantly lowered, allowing for broader access to innovative treatments. Also, high participation in clinical trials has led to the standardisation of treatment protocols, playing a key role in the improved survival rates seen in paediatric cancers.18
Challenges and future directions
Despite the strides made in paediatric brain cancer clinical trials, facilitated by the collaborative efforts of the ABCM and the ANZCHOG CNS Tumour Group, the path forward is fraught with operational and logistical challenges. Resource constraints remain a significant hurdle, with about 67% of clinical research staff funding derived from external, non‐operational resources (ANZCHOG data), highlighting a dependency on philanthropy and external grants for sustainability.
The transient nature of funding, with a significant portion of clinical research associates and clinical research nurses operating on short term contracts, exacerbates the challenges of recruitment and retention, leading to an exodus to industry and hindering the agility and continuity of clinical trial operations. These challenges underscore the necessity for sustained and strategic funding mechanisms that go beyond the ABCM, to maintain the current momentum.
The need for diverse treatment options, particularly investigational therapies when standard care falls short, underscores the importance of sustained and increased investment in research and clinical trials. Such commitment is vital to fulfill community expectations for accessible clinical trials, especially for conditions with challenging prognoses, ensuring families can find hope without the burden of seeking treatments abroad. The achievements to date, underpinned by strategic collaboration and targeted funding, serve as an example of what is possible. To build on this progress, it is essential to navigate the existing challenges with innovative funding models, regulatory agility and strengthened international partnerships. The goal is to create a continuously funded ecosystem that not only advances paediatric brain cancer research but also inspires similar initiatives across the broader paediatric oncology community. This vision is both aspirational and achievable, with continuous investment acting as the catalyst for a new era of clinical trial success, ultimately transforming the lives of these children and families.
Box 1 – Summary of Australian Brain Cancer Mission (ABCM) grant opportunity for enhancing brain cancer clinical trials
|
ABCM |
Details |
||||||||||||||
|
|
|||||||||||||||
|
Overview and purpose |
|
||||||||||||||
|
Funding and resources |
|
||||||||||||||
|
Objectives |
|
||||||||||||||
|
Expected outcomes |
|
||||||||||||||
|
Collaboration and capacity |
|
||||||||||||||
|
Infrastructure and support |
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Key stages in the Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) clinical trial development pipeline
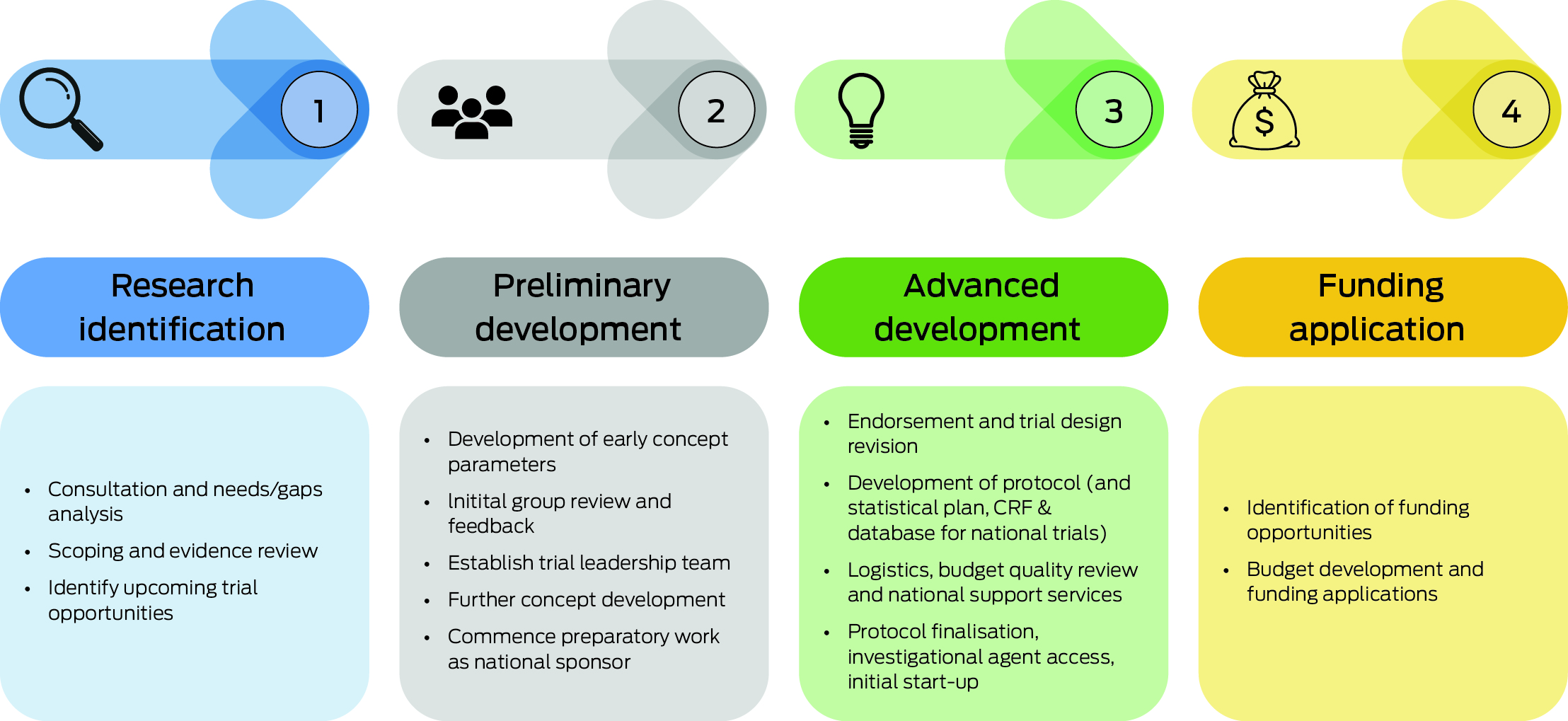
CRF = case report form. The pipeline consists of four stages: 1) research identification, involving setting priorities, consultations and forming partnerships; 2) preliminary development, where trial concepts are developed with input from international alliances and teams; 3) advanced clinical trial development, through a review process and support from the Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) National Trials Office; and 4) funding application development, aimed at identifying funding sources and supporting grant applications, with the addition of Australian Brain Cancer Mission (ABCM) funding to facilitate trial activities. This structure reflects ANZCHOG's approach to developing clinical trials underpinned by ABCM funding.
Box 3 – Paediatric brain cancer trials opened by Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) since the launch of the Australian Brain Cancer Mission (ABCM)
|
Trial with identifier |
Description and international sponsor |
||||||||||||||
|
|
|||||||||||||||
|
SJ‐ELiOT (NCT04023669) |
A phase 1/1b trial, born out of pre‐clinical research undertaken in Perth, Western Australia, for relapsed medulloblastoma patients.17 This multicentre collaboration involves St. Jude Children's Research Hospital (Tennessee, US), the German Cancer Research Centre (DKFZ), and the ANZCHOG Central Nervous System (CNS) Tumour Group. |
||||||||||||||
|
COZMOS trial (NCT03206021) |
Targeting epigenetic modifications to improve chemotherapy responsiveness in treatment‐resistant tumours, this initiative is a result of international collaboration with the Hospital for Sick Children in Toronto, Canada. |
||||||||||||||
|
SickKids Hypermutant Cancers study (NCT02992964) |
Focuses on immunotherapy for relapsed hypermutant cancers, including brain cancer. ANZCHOG CNS Tumour Group contributed to foundational research and is an active participant in this extended study in collaboration with the Hospital for Sick Children in Toronto, Canada. |
||||||||||||||
|
OZM‐063 LGG – Avastin (NCT02840409) |
A phase 2 trial for paediatric patients with low‐grade gliomas using anti‐angiogenic therapy in conjunction with standard chemotherapy, initiated by the Hospital for Sick Children in Toronto, Canada. |
||||||||||||||
|
INFORM2 NivEnt (NCT03838042) |
Incorporating immunotherapy and histone deacetylase inhibition for high risk refractory malignancies, initiated by the DKFZ. The first global enrolment was through our Australian sites. |
||||||||||||||
|
ReRAD (NCT03126266) |
Aiming to improve the quality of life for children with terminal diffuse intrinsic pontine glioma via re‐irradiation, initiated by University of Calgary, Canada. |
||||||||||||||
|
Met Med Can (NCT05230758) |
Examining metformin's cognitive and neurological benefits for paediatric medulloblastoma survivors. A multisite, double‐blind, placebo‐controlled trial initiated by the Hospital for Sick Children in Toronto, Canada. |
||||||||||||||
|
CONNECT 1903 (NCT04655404) |
A pilot study evaluating larotrectinib for paediatric patients with newly diagnosed high‐grade gliomas harbouring neurotrophic tropomyosin kinase receptors (NTRK) gene fusions, initiated by the Collaborative Network for NEuro‐oncology Clinical Trials (CONNECT) Consortium. |
||||||||||||||
|
PNOC019 (NCT04323046) |
A phase 1 study evaluating immunotherapy before and after surgical intervention in recurrent or progressive high‐grade gliomas, initiated by the Pediatric Neuro‐Oncology Consortium (PNOC). |
||||||||||||||
|
PNOC022 (NCT05009992) |
Investigating combination therapies for diffuse midline gliomas, including diffuse intrinsic pontine gliomas (DIPG), initiated by PNOC. |
||||||||||||||
|
TiNT (ACTRN12620001229965) |
Australian and New Zealand‐led study investigating trametinib therapy for children with neurofibromatosis type 1 (NF1), focusing not only on tumour response but also potential neurocognitive benefits. |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Currell A, Henshaw C. A Summary of childhood cancer statistics in Australia, 1983‐2020. Cancer Council Queensland, 2023. https://cancerqld.org.au/wp‐content/uploads/2023/08/23076_ACCR_Statistics_Summary_final.pdf (viewed Aug 2023).
- 2. Australian Institute of Health and Welfare. Australia's children, Cancer incidence and survival [website]. AIHW, 2022. https://www.aihw.gov.au/reports/children‐youth/australias‐children/contents/health/cancer‐incidence‐survival (viewed Aug 2023).
- 3. Pugh G, Bradbeer P, Wood A, et al. Childhood cancer incidence and survival in Aotearoa, New Zealand 2010‐2019. Cancer Epidemiol 2023; 86: 102433.
- 4. Kulubya ES, Kercher MJ, Phillips HW, et al. Advances in the treatment of pediatric brain tumors. Children (Basel) 2022; 10: 62.
- 5. Brinkman TM, Krasin MJ, Liu W, et al. Long‐term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: results from the St Jude Lifetime Cohort Study. J Clin Oncol 2016; 34: 1358‐1367.
- 6. Brinkman TM, Ness KK, Li Z, et al. Attainment of functional and social independence in adult survivors of pediatric CNS tumors: a report from the St Jude Lifetime Cohort Study. J Clin Oncol 2018; 36: 2762‐2769.
- 7. Stead M, Cameron D, Lester N, et al. Strengthening clinical cancer research in the United Kingdom. Br J Cancer 2011; 104: 1529‐1534.
- 8. O'Leary M, Krailo M, Anderson JR, et al. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol 2008; 35: 484‐493.
- 9. European Society for Paediatric Oncology. SIOP Europe strategic plan: update (2021‐2026). SIOP, 2021. https://siope.eu/media/documents/siop‐europes‐strategic‐plan‐update‐2021‐2026.pdf (viewed Aug 2023).
- 10. Australian Government, Cancer Australia. Australian Brain Cancer Mission [website]. Australian Government, 2023. https://www.canceraustralia.gov.au/key‐initiatives/australian‐brain‐cancer‐mission (viewed Oct 2024).
- 11. The Centre for International Economics. Review of the Australian Brain Cancer Mission. CIE, 2023. https://www.health.gov.au/resources/publications/review‐of‐the‐australian‐brain‐cancer‐mission?language=en (viewed Feb 2024).
- 12. Capper D, Jones DTW, Sill M, et al. DNA methylation‐based classification of central nervous system tumours. Nature 2018; 555: 469‐474.
- 13. Sturm D, Capper D, Andreiuolo F, et al. Multiomic neuropathology improves diagnostic accuracy in pediatric neuro‐oncology. Nat Med 2023; 29: 917‐926.
- 14. Liu APY, Li BK, Pfaff E, et al. Clinical and molecular heterogeneity of pineal parenchymal tumors: a consensus study. Acta Neuropathol 2021; 141: 771‐785.
- 15. Gajjar A, Robinson GW, Smith KS, et al. Outcomes by clinical and molecular features in children with medulloblastoma treated with risk‐adapted therapy: results of an international phase III trial (SJMB03). J Clin Oncol 2021; 39: 822‐835.
- 16. Bouffet E, Hansford JR, Garre ML, et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N Engl J Med 2023; 389: 1108‐1120.
- 17. Endersby R, Whitehouse J, Pribnow A, et al. Small‐molecule screen reveals synergy of cell cycle checkpoint kinase inhibitors with DNA‐damaging chemotherapies in medulloblastoma. Sci Transl Med 2021; 13: eaba7401.
- 18. Adamson PC. Improving the outcome for children with cancer: development of targeted new agents. CA Cancer J Clin 2015; 65: 212‐220.






Funding bodies and organisations that played a critical role in the successful execution of these studies: SJ Eliot (funded by the Medical Research Future Fund (MRFF), Carries Beanies 4 Brain Cancer, and ABCM), COZMOS (funded by the MRFF, Carries Beanies 4 Brain Cancer, and ABCM), Hypermutant HSC (funded by Cure Brain Cancer Foundation, and ABCM), LGG‐Avastin (funded by Cancer Australia), Inform2 (funded by MRFF, Paediatrio, Minderoo Foundation, and ABCM), ReRad (funded by ABCM), MetMed (funded by MRFF and ABCM), CONNECT1903 (funded by MRFF, The Kids Cancer Project (TKCP), and ABCM), PNOC019 (funded by Cure Brain Cancer Foundation, Robert Connor Dawes Foundation, and ABCM), PNOC022 (funded by Robert Connor Dawes Foundation, Isabella and Marcus Foundation, and ABCM), TiNT (funded by MRFF, Flicker of Hope, and Children's Tumour Foundation). This work has been made possible through these strategic partnerships, which drive both scientific innovation and clinical advancement in paediatric oncology. We value the instrumental role each entity has played in propelling our trial efforts forward, and we look to the future with optimism for the transformative outcomes that such collaborations promise. Jordan Hansford is funded by the Hospital Research Foundation and the Jamie McClurg Foundation. Nicholas Gottardo is funded by the Perth Children's Hospital Foundation Stan Perron Chair in Paediatric Oncology and Haematology. Raelene Endersby has support from a Cancer Council of Western Australia Research Fellowship and a Brainchild Fellowship from the Pirate Ship Foundation.
No relevant disclosures.