Aboriginal and Torres Strait Islander peoples proudly celebrate some of the world's oldest continuous cultures. However, our communities still bear the harsh and ongoing effects of colonisation, dispossession and racism, which continue to have a profound impact on our lives and our health outcomes. Despite increased focus on Aboriginal and Torres Strait Islander health by governments, mainstream health care providers and researchers, disparate health outcomes for Aboriginal and Torres Strait Islander peoples persist and culminate in a life expectancy that is more than eight years below the national average.1 The divide in cancer health outcomes between Aboriginal and Torres Strait Islander peoples and non‐Indigenous Australians runs equally deep — it likely reflects cancer health care that may not be fit‐for‐purpose to respond to the needs of Aboriginal and Torres Strait Islander peoples affected by cancer.
Precision medicine is becoming an increasingly important part of Australian health care, including in the context of cancer. Genomic screening can aid in cancer prevention and early detection, including cascade genomic testing of family members of individuals with cancer. Tumour molecular profiling is frequently employed in the clinic to more accurately delineate personalised treatment targets and choices for each individual according to their inherited and/or tumour genetic profile. Precision medicine holds promise to deliver greater cancer health equity through personalising cancer care to each individual. However, many questions of theoretical, practical, methodological and ethical significance remain unanswered. What is required to ensure that precision cancer medicine helps to address, rather than contribute to, the cancer health inequities experienced by Aboriginal and Torres Strait Islander peoples?
This article emerged through discussion between the authors as Aboriginal people working in the fields of Indigenous genomics and precision cancer research. Justine Clark is an Adnyamathanha woman and precision cancer researcher with a focus on improving cancer outcomes for Aboriginal and Torres Strait Islander peoples. Jessica Buck is a Kamilaroi woman and childhood cancer researcher investigating more effective and less toxic brain tumour treatments. Amanda Richards‐Satour is an Adnyamathanha and Barnagarla woman working on engaging with Aboriginal communities about genomics research. Louise Lyons is a Jaadwa woman with extensive experience in the health sector working across mainstream and Aboriginal community‐controlled health services in senior research, strategic and management roles. Alex Brown is a Yuin man and senior researcher with extensive experience in public health services, infectious diseases and chronic disease care, health care policy, and research.
Here, we contextualise precision cancer medicine for Aboriginal and Torres Strait Islander peoples by describing the broader landscape of cancer research and policy. We consider this critical background and write from our diverse cultural and professional perspectives to outline ways forward to ensuring safety, equity and benefit in precision cancer medicine for our communities. This narrative review puts forward a set of requirements, designed from an Aboriginal world view, to inform and guide the health professions, researchers and funders as they navigate the emerging area of precision cancer medicine as it applies to Aboriginal and Torres Strait Islander peoples.
Sources and selection criteria
Using a PubMed search including combinations of “Aboriginal”, “Indigenous”, “cancer”, “outcomes” and “Australia”, we conducted a review of publications from 2013 to 2023. We included seminal papers in the area, as well as key policy documents, nationwide cancer statistics reports, and health systems‐led Aboriginal cancer projects (Supporting Information).
The landscape of Aboriginal and Torres Strait Islander cancer research and policy
Defining cancer health disparities among Aboriginal and Torres Strait Islander peoples
Cancer is the leading broad cause of death for Aboriginal and Torres Strait Islander peoples,2 who are 40% more likely to die of cancer compared with non‐Indigenous Australians.3 Poorer five‐year survival rates for Aboriginal and Torres Strait Islander peoples compared with other Australians exist across almost all common cancer types.4 Overall cancer incidence is 14% higher for Aboriginal and Torres Strait Islander peoples compared with other Australians, with an increased incidence of cancers including those of the lung, head and neck, liver, cervix, and cancer of unknown primary site.4,5 Aboriginal and Torres Strait Islander peoples are more likely to be diagnosed with cancer at younger ages than non‐Indigenous Australians.5 Aboriginal and Torres Strait Islander peoples are less likely to access cancer screening services, which enable early diagnosis for some cancers,6 and, correspondingly, Aboriginal and Torres Strait Islander peoples typically present at a later stage of disease at diagnosis.5,7,8 Aboriginal and Torres Strait Islander peoples with cancer often have more comorbid conditions compared with non‐Indigenous Australians,5,9 with the most prevalent comorbid conditions among Aboriginal cohorts being diabetes and cardiovascular and respiratory diseases.10,11,12,13 Participation in cancer clinical trials remains inequitable, thought to be affected by the increased presence of comorbid conditions, geographic location, and experiences of racism and discrimination within the health system, factors that disproportionately affect Aboriginal and Torres Strait Islander populations.14,15
Interviews with Aboriginal and Torres Strait Islander cancer patients and health care workers identified an ongoing need for more culturally safe, competent and coordinated care.16,17 Aboriginal and Torres Strait Islander peoples still experience unacceptable levels of racism and discrimination within the health system; the proportion of Indigenous Australians who reported racial discrimination by doctors, nurses and/or medical staff has increased since 2014 (from 11% in 2014 to 20% in 2022).21 Aboriginal and Torres Strait Islander care coordination between service providers and jurisdictions could improve the provision of care for Aboriginal and Torres Strait Islander peoples from timely diagnosis to receipt of adequate treatment and follow‐up, particularly for those living remotely who must travel away from their home community or Country for treatment.18,19,20 In addition, it could play a role in supporting patients when issues of cultural safety intersect with often profound logistical challenges.
Aboriginal and Torres Strait Islander leadership in cancer control design and delivery
Aboriginal and Torres Strait Islander cancer control has become a national commitment embodied within two new cancer plans: the National Aboriginal Community Controlled Health Organisation (NACCHO) Aboriginal and Torres Strait Islander Cancer Plan (2023), and the Australian Cancer Plan.21,22 Both plans emphasise the development of Aboriginal and Torres Strait Islander community partnerships, building an Aboriginal and Torres Strait Islander cancer workforce, enshrining cultural safety within cancer services and utilising Aboriginal Community Controlled Health Organisations (ACCHOs) in care delivery as a trusted source of primary care. The Beautiful Shawl Project is a partnership between the Victorian Aboriginal Community Controlled Health Organisation (VACCHO) and BreastScreen Victoria.23,24 It has successfully implemented a bespoke community‐codesigned breast screening service for Aboriginal women that has seen more than 1000 breast screens delivered, with 40% of the participating women never having screened before and 29% who were late screeners.23,24 Other studies demonstrate that utilisation of ACCHOs in the provision of bowel and cervical cancer screening increased screening uptake among Aboriginal and Torres Strait Islander populations, who are typically underscreened.25,26,27 This offers insight into what is needed to deliver culturally safe and effective cancer control for Aboriginal and Torres Strait Islander populations at scale: Aboriginal and Torres Strait Islander leadership and codesign, and significant involvement of ACCHOs in care delivery.21,24
Towards precision cancer medicine for Aboriginal and Torres Strait Islander peoples
Population‐specific precision cancer medicine
Significant linguistic diversity exists among Aboriginal and Torres Strait Islander peoples, with over 250 distinct languages spoken at the time of colonisation and over 120 still spoken today,28 reflecting distinct population groups with unique cultural identities. Recent genomic analysis of Aboriginal and Torres Strait Islander peoples from four regions in northern and central Australia demonstrated genomic diversity between language groups and in a global context.29,30 Importantly, of the biallelic single nucleotide variants seen in this cohort, 26% were not observed within the Genome Aggregation Database (gnomAD).29 Furthermore, long‐read sequencing among individuals of the same Aboriginal and Torres Strait Islander cohort has identified large insertion–deletion variants and structural and copy number variants, of which many (up to 62%) were not previously annotated.30 This is clinically relevant given that the absence of a genomic variant in datasets such as gnomAD impedes the ability of clinicians to identify potentially pathogenic variants for clinical diagnoses.31 Therefore, a lack of knowledge of Aboriginal and Torres Strait Islander genomics may lead to a reduced ability to identify which variants are disease‐causing among these populations, leading to the potential of both missed and false‐positive clinical diagnoses for individuals. Whole genome sequencing of a cohort of 500 Aboriginal Tiwi Islander peoples in a separate study indicated that every individual carried at least one clinically actionable genotype across 19 pharmacogenes.32 In particular, 41% of the Tiwi cohort were predicted to have impaired cytochrome P450 2D6 (CYP2D6) enzyme, a key metaboliser of many drugs including the breast cancer drug tamoxifen, with this frequency being significantly higher compared with other global populations.33 These studies demonstrate unique, novel genomic variation among Aboriginal and Torres Strait Islander peoples, with potential clinical relevance with respect to pathogenic variant identification and cancer drug metabolism. However, these populations remain absent or under‐represented in human reference genome resources, genomic studies, cancer studies, cancer cell lines, patient‐derived xenografts and cancer clinical trials.15,34,35
Although the potential benefits of genomic medicine are clear, genomics carries with it a legacy of harm for Indigenous peoples globally. Indigenous communities have continuously raised concerns with exploitation and a lack of agency when invited to participate in projects exploring global genomic diversity.36 Aboriginal and Torres Strait Islander communities rejected the Human Genome Diversity Project, citing these same concerns.37
Developing our knowledge of cancer biology and genomics among Aboriginal and Torres Strait Islander peoples combined with treatment‐specific outcomes could empower prevention, precision diagnostics and novel therapeutics for individuals and their families. Yet, if a target for treatment can be identified, access to appropriate treatments is required, including the necessary clinical trial architecture to match patients with novel treatments. Approaches to enable Aboriginal and Torres Strait Islander peoples to benefit from precision cancer medicine must consider these challenges in accessing cancer care as well as cultural and historical contexts; it is on this backdrop that we outline the following requirements (Box).
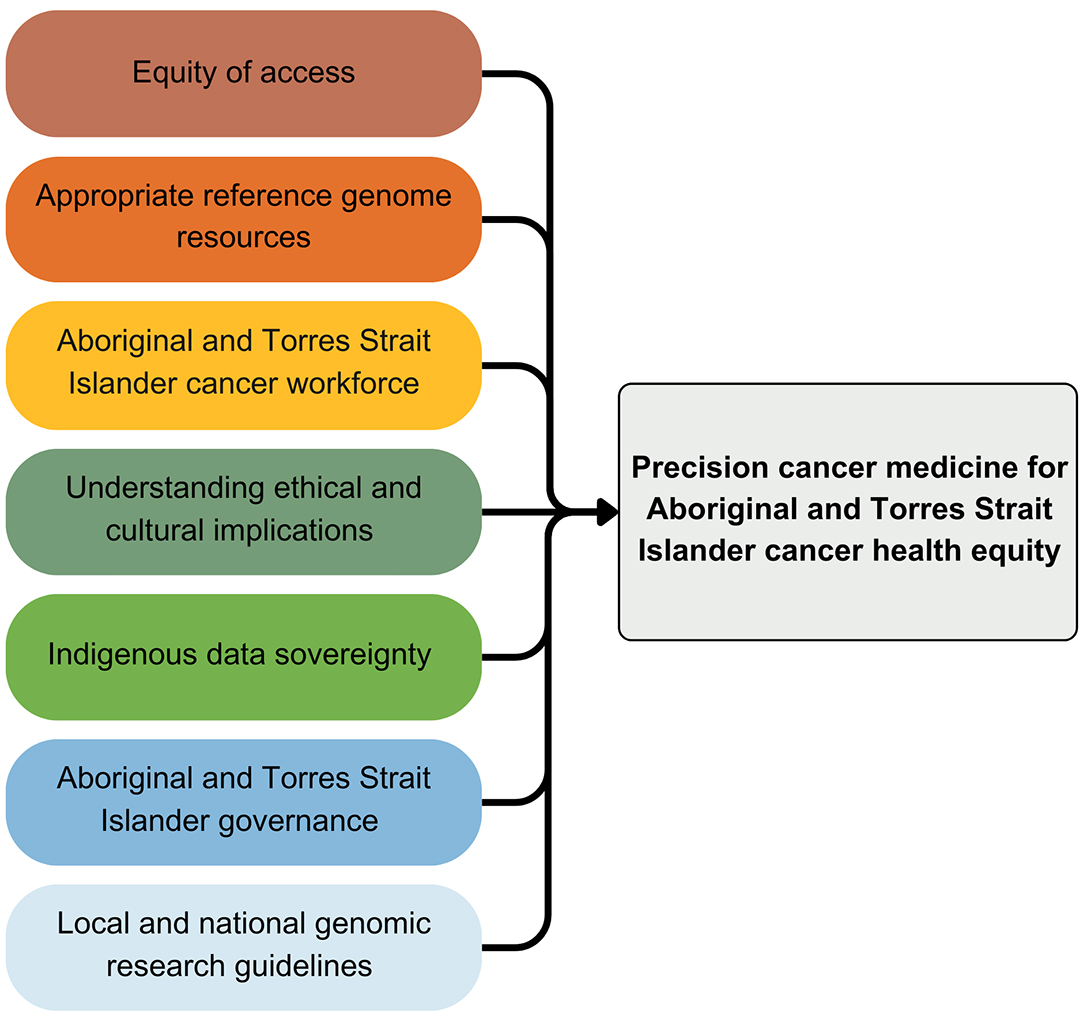
Requirements for precision cancer medicine for Aboriginal and Torres Strait Islander peoples
Equity of access to care. Equity of access clearly forms part of national and international agendas for Indigenous peoples living with cancer.22,38,39 All efforts to develop new interventions for Aboriginal and Torres Strait Islander peoples using cancer genomics must be inextricably linked to a robust public health systems approach, lest genomic medicine only serves to widen inequities. There is an immediate and ongoing need for public health systems and research centres to improve the cultural safety of their services and staff in line with the Closing the Gap Priority, Reform 3.40 In January 2024, there were 1585 cancer clinical trials registered on the Australian New Zealand Clinical Trials Registry (ANZCTR), of which only 17 trials (1.1%) were identified as having the words “Aboriginal”, “Indigenous” or “First Nations” included in the trial description or criteria (two duplicate records were removed from the analysis). Only one‐third (n = 6) of the 17 trials addressed the most prevalent cancers affecting Aboriginal and Torres Strait Islander people. Precision cancer researchers and oncologists have a responsibility to ensure that Aboriginal and Torres Strait Islander peoples have equity of access to clinical trials and have provided appropriate consent (including exploring culturally appropriate forms of consent). It is through clinical trials that patients with cancer can access new or repurposed cancer drugs, additional treatments after existing lines of treatment have been exhausted, and best practice clinical care and monitoring.41 More trials should focus on Aboriginal and Torres Strait Islander peoples as the priority cohort or a significant subcohort, which necessitates specific considerations within trial design that address existing barriers to access and enable individual and community benefit. Furthermore, the Indigenous status of cancer clinical trial participants should form a key component of routine trial participation documentation.
Appropriate genome reference resources. Ancestrally appropriate genome reference resources and background variant databases are required to ensure accuracy of interpretation of cancer genetic sequencing for Aboriginal and Torres Strait Islander peoples. Genome reference resources for precision cancer care are utilised across the cancer care continuum, from enabling accurate screening, cascade testing of family members, early detection and appropriate treatment decisions. The Silent Genomes project in Canada and the Aotearoa Variome project in New Zealand aim to produce Indigenous background variant databases to improve precision diagnostics for Indigenous populations locally.42 The Human Pangenome Reference Consortium has begun work to produce an updated human reference genome that better represents global genomic diversity,43 although this does not include Aboriginal and Torres Strait Islander individuals. Importantly, the genomic variation of Aboriginal and Torres Strait Islander peoples across language groups is indicative that a single Aboriginal and Torres Strait Islander reference genome or background variant database may not be sufficient without wide language group representation.29 The National Centre for Indigenous Genomics has recently received funding to build a reference genome from samples of geographically dispersed Aboriginal and Torres Strait Islander peoples predominantly across the Northern Territory, Queensland and Western Australia.44
Aboriginal and Torres Strait Islander cancer workforce. An essential component of strategies to deliver the benefit of precision cancer medicine for Aboriginal and Torres Strait Islander communities is through building a workforce of Aboriginal and Torres Strait Islander medical, surgical and radiation oncologists, cancer nurses, care coordinators, genetic counsellors, researchers and bioinformaticians. Further to this, directly supporting the existing Aboriginal health workforce in understanding and implementing precision cancer care. In particular, numerous studies advocate for Aboriginal‐led care coordination.10,17,18,19,20 Ivers and colleagues45 found that an Aboriginal cancer care team provided culturally safe care coordination that helped Aboriginal patients overcome barriers to receiving cancer care; there is a clear need to consider the expansion of these approaches locally and nationally. A substantial commitment toward Aboriginal and Torres Strait Islander cancer workforce development has been made within the Australian Cancer Plan, the NACCHO Aboriginal and Torres Strait Islander Cancer Plan and the 2023–2024 federal Budget.
Understanding of the ethical and cultural implications of precision cancer research. Precision cancer research often involves the functional validation of novel genomic variation in cancer using human samples. These human samples are often curated and stored long term in a biobank of tissue or other biological samples, with associated data for each sample often stored within a data registry. Immortalised human cancer cell lines or patient‐derived xenografts may then be generated and stored long term to study the effects of a gene of interest on cancer biology. Generation of bespoke cancer research tools to investigate Aboriginal and Torres Strait Islander cancer, their curation and long term storage will carry ethical and cultural implications for Aboriginal and Torres Strait Islander peoples that are not yet understood by the research community. Extensive community engagement to better understand these implications and identify the acceptable use of precision cancer research tools for Aboriginal and Torres Strait Islander peoples is essential. This is also necessary to understand the diversity of cultural views and practices relevant to particular language groups, patients, consumer or policy and health care stakeholders. Before the generation and curation of Aboriginal and Torres Strait Islander samples for the purposes of cancer research, community (often referred to as collective consent) alongside individual level consent may need to be obtained.
Indigenous data sovereignty. The ability to share genomics data is a core element of precision medicine initiatives, including within the context of precision oncology. However, data sharing guidelines and principles have largely ignored Indigenous peoples’ concerns, interests, perspectives and protocols.46,47 Global efforts are currently underway to develop data sharing guidelines that are more consistent with the needs of culturally diverse communities. The CARE principles (collective benefit, authority to control, responsibility, ethics) have been developed to align data sharing with Indigenous data sovereignty guidelines.48 The CARE principles should be enacted in the context of precision cancer medicine through purposeful design of data ecosystems to incorporate Aboriginal and Torres Strait Islander data governance structures and to enable Aboriginal and Torres Strait Islander peoples to derive collective benefit from their data.
Aboriginal and Torres Strait Islander governance in precision cancer research. Aboriginal and Torres Strait Islander governance structures must be developed and implemented to govern research projects from research priority and benefits generation to results analysis, interpretation and translation.49 In the specific context of precision cancer research and clinical trials, a robust governance framework should be developed at the national level and implemented at the level of each research project working with Aboriginal and Torres Strait Islander participants to protect Aboriginal and Torres Strait Islander genomic and health data. Moreover, to ensure that precision cancer research is purposeful, meets the demonstrated need to close the gap in cancer outcomes for Aboriginal and Torres Strait Islander communities, and meets best practice ethical guidelines, the parameters of this research must be defined by communities. Researchers should seek to build partnerships and empower communities in research through capacity building in genomics and precision medicine, ongoing reciprocity and community level accountability and evaluation.
Local and national genomic research guidelines. Although the guiding principles of Aboriginal and Torres Strait Islander health research are already outlined within specific guidelines,49,50 the interpretation of these as they relate to the unique ethical considerations for genomics and precision cancer research is less clear. Local genomic research guidelines have been developed for research with Aboriginal and Torres Strait Islander communities in Queensland following extensive community consultation.51,52 Such guidelines are needed for each Australian jurisdiction, and nationally, to provide researchers, ethics committees, and communities with an outline of the expectations of all parties involved and to act as an additional accountability mechanism for genomics research.
Conclusion
Aboriginal and Torres Strait Islander peoples are disproportionately affected by cancer and face barriers to accessing standard as well as precision cancer diagnostics, research and care. We have outlined a set of requirements for the translation of precision cancer research into meaningful outcomes for Aboriginal and Torres Strait Islander peoples. Aboriginal and Torres Strait Islander communities have governed and protected their health and wellbeing for thousands of generations — defining safety, benefit and sovereignty in precision medicine research for their communities should rest in their hands.
Provenance: Not commissioned; externally peer reviewed.
- Justine R Clark (Adnyamathanha)1,2
- Jessica Buck (Kamilaroi)3,4
- Amanda Richards‐Satour (Adnyamathanha and Barngarla)1
- Louise Lyons (Jaadwa)1
- Alex Brown (Yuin)1,5
- 1 Telethon Kids Institute, Adelaide, SA
- 2 Australian National University, Canberra, ACT
- 3 Telethon Kids Cancer Centre, Telethon Kids Institute, Perth, WA
- 4 Centre for Child Health Research, University of Western Australia, Perth, WA
- 5 National Centre for Indigenous Genomics, Australian National University, Canberra, ACT
Open access:
Open access publishing facilitated by Australian National University, as part of the Wiley – Australian National University agreement via the Council of Australian University Librarians.
No relevant disclosures.
- 1. Australian Bureau of Statistics. Aboriginal and Torres Strait Islander life expectancy [reference period 2020–2022]. Canberra: ABS, 2023. https://www.abs.gov.au/statistics/people/aboriginal‐and‐torres‐strait‐islander‐peoples/aboriginal‐and‐torres‐strait‐islander‐life‐expectancy/latest‐release (viewed Dec 2023).
- 2. Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander Health Performance Framework — summary report 2024. Canberra: AIHW, 2024. https://www.indigenoushpf.gov.au/report‐overview/overview/summary‐report (viewed Dec 2023).
- 3. Australian Institute of Health and Welfare. Cancer in Aboriginal and Torres Strait Islander peoples of Australia: an overview [Cat. No. CAN 75]. Canberra: AIHW, 2013. https://www.aihw.gov.au/reports/cancer/cancer‐in‐indigenous‐australians‐overview/summary (viewed Dec 2023).
- 4. Australian Institute of Health and Welfare. Cancer in Australia 2021 [Cat. No. CAN 144]. Canberra: AIHW, 2021. https://www.aihw.gov.au/reports/cancer/cancer‐in‐australia‐2021 (viewed Dec 2023).
- 5. Banham D, Roder D, Keefe D, et al. Disparities in cancer stage at diagnosis and survival of Aboriginal and non‐Aboriginal South Australians. Cancer Epidemiol 2017; 48: 131‐139.
- 6. Banham D, Roder D, Keefe D, et al. Disparities in breast screening, stage at diagnosis, cancer treatment and the subsequent risk of cancer death: a retrospective, matched cohort of aboriginal and non‐aboriginal women with breast cancer. BMC Health Serv Res 2019; 19: 387.
- 7. Fitzadam S, Lin E, Creighton N, et al. Lung, breast and bowel cancer treatment for Aboriginal people in New South Wales: a population‐based cohort study. Intern Med J 2021; 51: 879‐890.
- 8. Gibberd A, Supramaniam R, Dillon A, et al. Are Aboriginal people more likely to be diagnosed with more advanced cancer? Med J Aust 2015; 202: 195‐199. https://www.mja.com.au/journal/2015/202/4/are‐aboriginal‐people‐more‐likely‐be‐diagnosed‐more‐advanced‐cancer#:~:text=While%20for%20all%20cancers%20combined,disease%20at%20diagnosis%20(Appendix)
- 9. Tervonen HE, Morrell S, Roder D, et al. Differences in cancer incidence by age at diagnosis between Aboriginal and non‐Aboriginal people for cancer types included in Australian national screening programs. Cancer Epidemiol 2019; 60: 102‐105.
- 10. Diaz A, Moore SP, Martin JH, et al. Comorbidities amongst Indigenous cancer patients: impact on treatment and survival. Int J Epidemiol 2015; 44(Suppl): i2‐i3.
- 11. Weir K, Supramaniam R, Gibberd A, et al. Comparing colorectal cancer treatment and survival for Aboriginal and non‐Aboriginal people in New South Wales. Med J Aust 2016; 204: 156. https://www.mja.com.au/journal/2016/204/4/comparing‐colorectal‐cancer‐treatment‐and‐survival‐aboriginal‐and‐non‐aboriginal
- 12. Pule L, Buckley E, Niyonsenga T, et al. Developing a comorbidity index for comparing cancer outcomes in Aboriginal and non‐Aboriginal Australians. BMC Health Serv Res 2018; 18: 776.
- 13. Rodger JC, Supramaniam R, Gibberd AJ, et al. Prostate cancer mortality outcomes and patterns of primary treatment for Aboriginal men in New South Wales, Australia. BJU Int 2015; 115: 16‐23.
- 14. Jessop S, Ruhayel S, Sutton R, et al. Are outcomes for childhood leukaemia in Australia influenced by geographical remoteness and Indigenous race? Pediatr Blood Cancer 2021; 68: e28945.
- 15. Cunningham J, Garvey G. Are there systematic barriers to participation in cancer treatment trials by Aboriginal and Torres Strait Islander cancer patients in Australia? Aust N Z J Public Health 2021; 45: 39‐45.
- 16. Anderson K, Diaz A, Parikh DR, Garvey G. Accessibility of cancer treatment services for Indigenous Australians in the Northern Territory: perspectives of patients and care providers. BMC Health Serv Res 2021; 21: 95.
- 17. Jessop S, Hill S, Bicanin K, et al. Aboriginal children with cancer: the patient and healthcare worker perspective. Pediatr Blood Cancer 2024; 71: e30747.
- 18. Whop LJ, Valery PC, Beesley VL, et al. Navigating the cancer journey: a review of patient navigator programs for Indigenous cancer patients. Asia Pac J Clin Oncol 2012; 8: 89‐96.
- 19. Corbett CM, Somers TJ, Nuñez CM, et al. Evolution of a longitudinal, multidisciplinary, and scalable patient navigation matrix model. Cancer Med 2020; 9: 3202‐3210.
- 20. Reilly R, Micklem J, Yerrell P, et al. Aboriginal experiences of cancer and care coordination: lessons from the Cancer Data and Aboriginal Disparities (CanDAD) narratives. Health Expect 2018; 21: 927‐936.
- 21. National Aboriginal Community Controlled Health Organisation. NACCHO Aboriginal and Torres Strait Islander Cancer Plan. Canberra: NACCHO, 2023. https://www.naccho.org.au/cancer/ (viewed Jan 2024).
- 22. Cancer Australia. Australian Cancer Plan. Sydney: Cancer Australia, 2023. https://www.australiancancerplan.gov.au/welcome (viewed Dec 2023).
- 23. Victorian Aboriginal Community Controlled Health Organisation. The Beautiful Shawl: implementation model. Melbourne: VACCHO, 2022. https://clara.breastscreen.org.au/intranet/documents/11/3800/The_Beautiful_Shawl_Implementation_Model_Apr2022.pdf (viewed Dec 2023).
- 24. Victorian Aboriginal Community Controlled Health Organisation. Victorian Aboriginal Cancer Journey Strategy. Melbourne: VACCHO, 2023. https://www.vaccho.org.au/wp‐content/uploads/2023/05/VACCHO_Victorian‐Aboriginal‐Cancer‐Journey‐Strategy‐2023‐2028_Web.pdf (viewed Dec 2023).
- 25. Dasgupta P, Condon JR, Whop LJ, et al. Access to aboriginal community‐controlled primary health organizations can explain some of the higher Pap test participation among Aboriginal and Torres Strait Islander women in North Queensland, Australia. Front Oncol 2021; 11: 725145.
- 26. Menzies School of Health Research. National Indigenous bowel screening pilot — final report, October 2020. Darwin: Menzies School of Health Research, 2020. https://www.health.gov.au/sites/default/files/documents/2021/06/final‐report‐on‐the‐national‐indigenous‐bowel‐screening‐pilot.pdf (viewed Dec 2023).
- 27. National Aboriginal Community Controlled Health Organisation. Annual report 2022–2023. Canberra: NACCHO, 2023. https://www.naccho.org.au/publications‐resources/ (viewed Jan 2024).
- 28. Marmion D, Obata K, Troy J. Community, identity, wellbeing: the report of the Second National Indigenous Languages Survey. Canberra: Australian Institute Aboriginal and Torres Strait Islander Studies, 2014. https://aiatsis.gov.au/sites/default/files/2020‐09/2014‐report‐2nd‐national‐indigenous‐languages‐survey.pdf (viewed Jan 2024).
- 29. Silcocks M, Farlow A, Hermes A, et al. Indigenous Australian genomes show deep structure and rich novel variation. Nature 2023; 624: 593‐601.
- 30. Reis ALM, Rapadas M, Hammond JM, et al. The landscape of genomic structural variation in Indigenous Australians. Nature 2023; 624: 602‐610.
- 31. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405‐424.
- 32. Samarasinghe SR, Hoy W, Jadhao S, et al. The pharmacogenomic landscape of an Indigenous Australian population. Front Pharmacol 2023; 14: 1180640.
- 33. Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther 2018; 103: 770‐777.
- 34. Schneider VA, Graves‐Lindsay T, Howe K, et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res 2017; 27: 849‐864.
- 35. Guerrero S, López‐Cortés A, Indacochea A, et al. Analysis of racial/ethnic representation in select basic and applied cancer research studies. Sci Rep 2018; 8: 13978.
- 36. Fox K. The illusion of inclusion — the “All of Us” research program and Indigenous peoples’ DNA. N Engl J Med 2020; 383: 411‐413.
- 37. Dodson M, Williamson R. Indigenous peoples and the morality of the Human Genome Diversity Project. J Med Ethics 1999; 25: 204‐248.
- 38. Ministry of Health. New Zealand Cancer Action Plan 2019–2029 — Te Mahere mō te Mate Pukupuku o Aotearoa 2019–2029. Wellington: Ministry of Health, 2020. https://www.health.govt.nz/publication/new‐zealand‐cancer‐action‐plan‐2019‐2029 (viewed Dec 2023).
- 39. Cancer Care Ontario, Ontario Health. First Nations, Inuit, Métis and Urban Indigenous Cancer Strategy 2019–2023. Ontario: Ontario Health, 2019. https://www.cancercareontario.ca/en/cancer‐care‐ontario/programs/aboriginal‐programs/indigenous‐cancer‐strategy (viewed Jan 2024).
- 40. Joint Commission on Closing the Gap. National Agreement on Closing the Gap. Canberra: Joint Commission on Closing the Gap, 2020. https://www.closingthegap.gov.au/national‐agreement (viewed Jan 2024).
- 41. Bouzalmate‐Hajjaj A, Massó Guijarro P, Khan KS, et al. Benefits of participation in clinical trials: an umbrella review. Int J Environ Res Public Health 2022; 19: 15368.
- 42. Caron NR, Chongo M, Hudson M, et al. Indigenous genomic databases: pragmatic considerations and cultural contexts. Front Public Health 2020; 8: 111.
- 43. Liao WW, Asri M, Ebler J, et al. A draft human pangenome reference. Nature 2023; 617: 312‐324.
- 44. National Health and Medical Research Council. An Indigenous Australian reference genome: Indigenous inclusion in the benefits of genomic medicine. Canberra: NHMRC, 2018. https://www.grants.gov.au/Ga/Show/A8935E11‐E7E8‐A9B6‐007A‐4422116C78EA (viewed May 2024).
- 45. Ivers R, Jackson B, Levett T, et al. Home to health care to hospital: evaluation of a cancer care team based in Australian Aboriginal primary care. Aust J Rural Health 2019; 27: 88‐92.
- 46. United Nations. United Nations Declaration on the Rights of Indigenous Peoples [A/RES/61/295]. New York: UN, 2007. https://www.un.org/development/desa/indigenouspeoples/wp‐content/uploads/sites/19/2018/11/UNDRIP_E_web.pdf (viewed May 2024).
- 47. Hudson M, Garrison NA, Sterling R, et al. Rights, interests and expectations: Indigenous perspectives on unrestricted access to genomic data. Nat Rev Genet 2020; 21: 377‐384.
- 48. Carroll SR, Garba I, Figueroa‐Rodríguez OL, et al. The CARE Principles for Indigenous data governance. Data Sci J 2020; 19: 43.
- 49. National Health and Medical Research Council. Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities. Canberra: NHMRC, 2018. https://www.nhmrc.gov.au/about‐us/resources/ethical‐conduct‐research‐aboriginal‐and‐torres‐strait‐islander‐peoples‐and‐communities (viewed Dec 2023).
- 50. Morey K, Franks C, Pearson O, et al. Research ACCORDing to whom? Developing a South Australian Aboriginal and Torres Strait Islander Health Research Accord. Lowitja Journal 2023; 1: 100003.
- 51. Kaladharan S, Vidgen ME, Pearson JV, et al. Ask the people: developing guidelines for genomic research with Aboriginal and Torres Strait Islander peoples. BMJ Glob Health 2021; 6: e007259.
- 52. QIMR Berghofer Medical Research Institute. Genomic partnerships: guidelines for genomic research with Aboriginal and Torres Strait Islander peoples of Queensland. Brisbane: QIMR Berghofer Medical Research Institute, 2019. https://www.qimrberghofer.edu.au/wp‐content/uploads/2020/09/2019‐Indigenous‐Health‐Genomics‐Guide‐v9‐WEB.pdf (viewed Jan 2024).






Summary