The known: Kidney failure has been increasing in Australia among Aboriginal and Torres Strait Islander children and young adults.
The new: Since 1963, we observed excellent 5‐year graft and recipient survivals among all children and young adults who achieved transplantation. We note, however, fewer transplants were achieved within the first five years of dialysis initiation among Aboriginal and Torres Strait Islander children and young adults, and their continuing on dialysis without transplant was associated with lower patient survival.
The implications: Models of care that achieve transplantation within five years of dialysis commencement must be prioritised for Aboriginal and Torres Strait Islander children and young adults.
Kidney failure or end‐stage kidney disease affects 5–15 children (age < 18 years) per million per year.1,2,3 Kidney transplantation is the optimal choice for children with end‐stage kidney disease; dialysis is usually prescribed only as a bridging therapy until the child receives a transplant. Kidney transplantation is associated with longer life expectancy, better quality of life, and lower costs than long term dialysis therapy.4 Ethnic group‐related differences in access to kidney transplantation for adults have been reported in the United States.5 Paediatric studies in the United States6 and Europe7 have similarly found that children and adolescents from Black communities and families have less access to waiting lists for deceased donor organs and lower rates of pre‐emptive and living donor transplantation than other children.
In Australia, Aboriginal and Torres Strait Islander adults have less access to kidney transplantation than non‐Indigenous people with end‐stage kidney disease.8,9 The major causes of chronic kidney disease in Aboriginal and Torres Strait Islander adults are not genetic, and while this is also true for non‐Indigenous Australians, the pervasive unchallenged systemised bias in health systems in Australia for more than 235 years10 has influenced survival and health care availability.
We have previously reported the rising incidence of end‐stage kidney disease among Aboriginal and Torres Strait Islander children and young adults and their poorer access to kidney transplantation than other children and young adults.11
We now compared Aboriginal and Torres Strait Islander with non‐Indigenous children and young adults to determine the extent to which health care models support their survival. We examined whether access to transplantation has been equitable for both groups, to support advocacy and care models that remedy inequity. We specifically examined access to kidney transplantation, graft survival, recipient survival after kidney transplantation (death with functioning graft), survival of children and young adults who remained on dialysis and did not receive kidney transplants, and causes of death and graft loss. Few studies have compared the equity of transplantation access and survival outcomes (person and graft) of Aboriginal and Torres Strait Islander with non‐Indigenous children and young adults in Australia.12 Our study provides evidence for informing models of care that advance treatment accessibility, quality of care, and health outcomes for Aboriginal and Torres Strait Islander children, young adults, and their families.
Methods
For our retrospective cohort study, we used data prospectively collected by the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA; https://www.anzdata.org.au/anzdata). ANZDATA is a clinical quality registry that collates accurate, appropriate, and comprehensive data kidney replacement therapy and kidney failure outcomes from all dialysis and renal units in Australia and New Zealand with the aim of improving quality of care and outcomes. We analysed de‐identified data on kidney replacement therapy for all people aged 0–24 years who commenced kidney replacement therapy in Australia during 1963–2020, including ethnic background, demographic characteristics, primary kidney disease, other medical conditions, and treatment modality.
Study outcomes
Primary outcomes were time to first kidney transplantation, death, death‐censored graft survival, and survival for people with end‐stage kidney disease who did not receive kidney transplants (ie, remained on dialysis) during 1963–2020. We compared outcomes for Aboriginal and Torres Strait Islander children and young adults with those for non‐Indigenous children and young adults. Time to first kidney transplantation was measured from the date of commencement of dialysis to the date of first transplantation, censored at the date of loss to follow‐up or 31 December 2020, whichever came first. Post‐transplantation survival was calculated from the date of transplantation to the date of death or that of the most recent follow‐up. Patient survival after transplant, means that while the graft initially survived and functioned, even if it failed at some later time, the patient remained alive (and can be calculated at a specific time, such as five or ten years). Death‐censored graft survival (graft survival) refers to the time for which a transplanted organ remains functional, excluding cases in which the recipients died. “Patient survival on dialysis,” without accessing transplantation, was measured from the date of commencement of dialysis to death, censored at loss to follow‐up or 31 December 2020, whichever came first. Survival on dialysis was considered for the whole period of 1963–2020. The first kidney transplant among non‐Indigenous children and young adults was 1963, and in 1971 for Aboriginal or Torres Strait Islander children and young adults. Therefore, reporting of graft survival and recipient survival was limited to 1970–2020.
Variables
The primary exposure was ethnic background (Aboriginal and Torres Strait Islander or non‐Indigenous). Variables examined as potential confounders were age at initiation of dialysis, gender, primary kidney disease, remoteness,13 cardiovascular disease (ischaemic heart disease, cerebrovascular disease, or peripheral vascular disease), diabetes mellitus, chronic lung disease, late referral to nephrologist (less than three months before initiation of kidney replacement therapy), state/territory of treatment, and era of kidney replacement therapy commencement (1970–1985, 1986–1997, 1998–2004, 2005–2020). People classified as having glomerulonephritis included those with familial and nonfamilial glomerulonephritis. Congenital malformation of the kidney and urinary tract refers to structural abnormalities present at birth affecting the kidneys, ureters, bladder, or urethra.
We undertook a complete case analysis, excluding the few people for whom data were missing for some variables. Transplantation characteristics (age at transplant, donor type, human leukocyte antigen [HLA] mismatch, panel‐reactive antibodies (PRA) score, cold ischaemia time, donor age, era) were also included as variables when assessing post‐transplantation recipient survival and death‐censored graft survival. Transplantation era was defined by the predominant baseline immunosuppression used: 1970–1985, azathioprine and prednisone; 1986–1997, cyclosporine/azathioprine/prednisone; 1998–2004, cyclosporine/mycophenolate/prednisone; 005–2020, tacrolimus/mycophenolate/prednisone.
“Non‐adherence with treatment regimens” is a categorical ANZDATA term defined by the clinician's assessment of the patient's inconsistency of use of transplantation clinical care. The status at modality treatment transition is defined as receiving a transplant, death, on dialysis, own kidney function recovered, or date of most recent visit if lost to follow‐up.
Statistical analysis
Statistical analyses were conducted in R 4.2.3 (R Foundation for Statistical Computing). Continuous variables are summarised as medians with interquartile ranges (IQRs); the statistical significance of differences by ethnic background were assessed in Mann–Whitney U tests. Categorical variables are summarised as counts and proportions; the statistical significance of differences by ethnic background were assessed in χ2 tests, with continuity correction when appropriate.
Survival outcomes (death‐censored graft survival, post‐transplantation survival, survival while on dialysis) were assessed in Cox proportional hazards models (using the coxph function in the survival package14). The association between ethnic background and survival was initially examined in univariate models. Multivariate models included covariates considered clinically important (age, gender, primary renal disease) or with statistically significant associations in the univariate models. Survival was estimated with the likelihood ratio method; we report adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). The proportional hazard assumption was tested by visually examining log‐log plots of survival and Schoenfeld residuals. Survival is depicted in Kaplan–Meier survival curves (generated using the ggsurvplot function in the survminer package15), and compared in log‐rank tests. P < 0.05 (2‐sided) was deemed statistically significant.
Positionality
Swasti Chaturvedi is a paediatric nephrologist and identifies as an Indian Australian; she is a senior staff specialist and clinician researcher at Sydney Children's Hospital, Sydney (Gadigal Country), since mid‐2023. She worked in Darwin (Larrakia Country), Northern Territory, during 2017–2023 where she initiated and led the Northern Territory paediatric nephrology service. Shahid Ullah is an Associate Professor in Biostatistics, with experience in clinical quality registry data reporting, and identifies as Bangladeshi Australian. Jaquelyne Hughes is a Torres Strait Islander woman (Wagadagam Tribe), and a nephrologist and clinician researcher, mentor, and Indigenous health systems innovator working on Larrakia Country, Darwin. The reporting of the study was guided by the Consolidated criteria for strengthening reporting of health research involving indigenous peoples (CONSIDER; Supporting Information).16
Ethics approval
The study was approved by the human research ethics committee of the Northern Territory Department of Health and Menzies School of Health Research (2020‐3324), and by the ANZDATA Aboriginal and Torres Strait Islander Health working group according to its Guidance on data requests relating to patient ethnicity, designed in consultation with Aboriginal and Torres Strait Islander people.17
Results
During 1963–2020, 3736 children and young adults received kidney replacement therapy in Australia; including 213 Aboriginal and Torres Strait Islander (5.8%; median age, 20 years; IQR, 15–22 years), and 3523 who were non‐Indigenous (94.2%; median age, 18 years; IQR, 13–22 years). Female participants accounted for 99 Aboriginal and Torres Strait Islander patients (46.5%) and 2111 non‐Indigenous patients (59.9%). The most frequent causes of end‐stage kidney disease were glomerulonephritis and congenital malformations of the kidney and urinary tract. Diabetes and hypertension were more commonly recorded as causes of end stage kidney failure in Aboriginal and Torres Strait Islander children and young adults (Box 1). A remote location of residence was more frequent among Aboriginal and Torres Strait Islander children and young adults (71, 37.6% v 44, 1.7%). Late referral to a nephrology service was recorded more frequently among Aboriginal and Torres Strait Islander children and young adults (57, 32.2% v 537, 20.4%) (Box 1).
Access to kidney transplantation
During follow‐up between 1970 and 2020 (median, eight years; IQR, 2.6–15 years), 2762 children and young adults (74%) received kidney transplants, of these 93 were Aboriginal and Torres Strait Islander children and young adults (3.4%) and 2669 were non‐Indigenous children and young adults (96.6%). The median age at transplantation was similar for Aboriginal and Torres Strait Islander (17 years; IQR, 13–21 years) and non‐Indigenous children and young adults (18 years; IQR, 12–22 years). A substantial difference in transplantation within five years was observed among Aboriginal and Torres Strait Islander compared with non‐Indigenous children and young adults (99, 46% v 2924, 83%; aHR, 0.39; 95% CI, 0.32–0.48). Furthermore, fewer Aboriginal and Torres Strait Islander children and young adults received living donor transplants (19, 20% v 1170, 43.9%), or underwent pre‐emptive transplantation (one, 1.1% v 363, 13.6%).
There were substantial differences in immune compatibility between the groups, with five or six HLA mismatches (36, 41% v 293, 13.4%), and high PRA scores in the range of 50–100% (13, 14% v 149, 5.8%) being more frequent among Aboriginal and Torres Strait Islander than non‐Indigenous children and young adults. Cold ischaemia time exceeding 18 hours was also more frequently observed in Aboriginal and Torres Strait Islander children and young adults: (26, 31% v 341, 16.6%). Differences of donor age and immunosuppression used (after adjustment for transplantation era) were not statistically significant (Box 2).
Graft survival
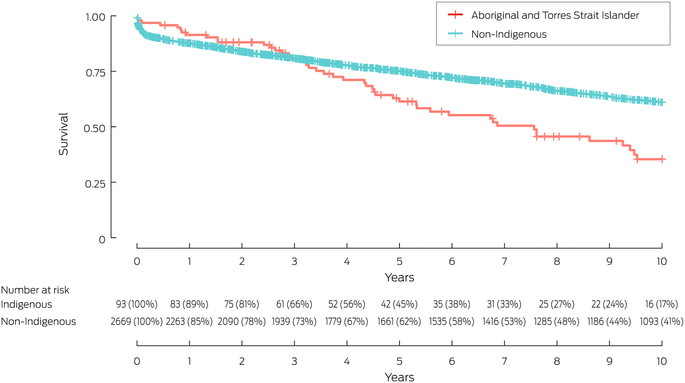
Survival was similar very early after transplant; however, it diverged from three years (Box 3). At five years, survival outcomes were 61% v 75%; (aHR, 1.43; 95% CI, 1.00–2.05) between Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults. Ten‐year graft survival was lower for Aboriginal and Torres Strait Islander children and young adults (35% v 61%; aHR, 1.69; 95% CI, 1.25–2.28) (Box 3).
The most frequent causes of graft failure in both Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults were chronic allograft nephropathy, acute rejection, and technical or vascular problems. Non‐adherence with post‐transplantation care leading to graft failure was more frequently recorded among Aboriginal and Torres Strait Islander children and young adults (12 of 53, 23% v 77 of 1383, 5.6%) (Box 4).
Survival after kidney transplantation
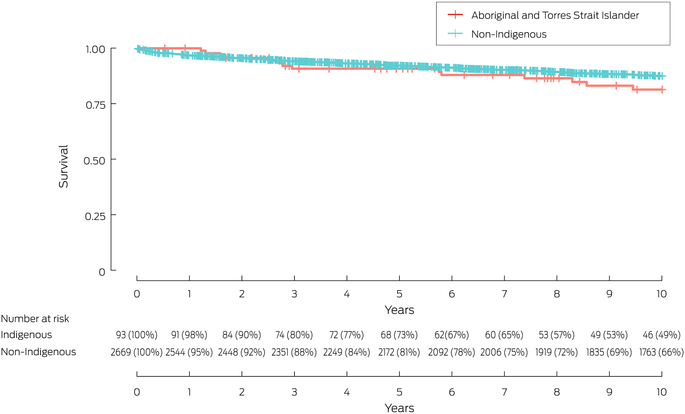
Five‐year survival after kidney transplantation was similar for Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults (91% v 92%; aHR, 1.15; 95% CI, 0.56–2.33), as was ten‐year survival (81% v 88%; aHR, 1.40; 95% CI, 0.82–2.41) (Box 5). The most frequent causes of death were cardiovascular (Aboriginal and Torres Strait Islander people: 13, 46%; non‐Indigenous people: 263, 33%) (Box 6).
Survival on dialysis without access to transplantation
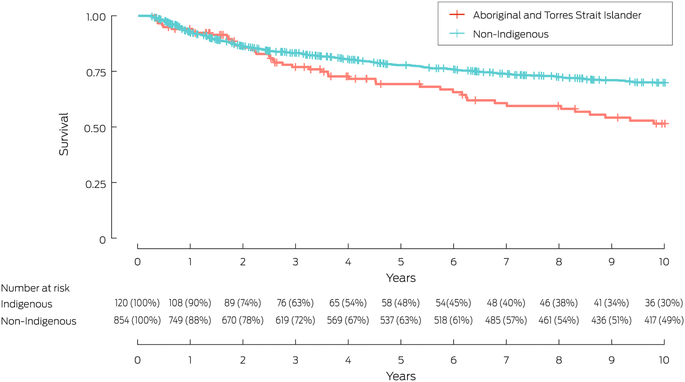
Five‐year survival of those who remained on dialysis (and did not access transplantation) was not statistically different for Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults (69% v 78%; aHR, 1.30; 95% CI, 0.87–1.92). At ten years, survival was poorer for Aboriginal and Torres Strait Islander than non‐Indigenous children and young adults (51% v 70%; aHR, 1.50; 95% CI, 1.08–2.10) (Box 7). The most frequent causes of death were cardiovascular (Aboriginal and Torres Strait Islander children and young adults: 37, 63%; and non‐Indigenous children and young adults: 144, 33%) (Box 8).
Discussion
This study examined graft and patient survival post‐transplant during 1963–2020, since being alive and alive without need for intensive dialysis treatment are important for children, young adults and their families. Since kidney transplantation is the internationally accepted treatment for children, our study reassuringly confirmed, for patient survival, that both Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults have excellent survival at five and ten years post transplantation. Despite this incredible survival outcome, the study also identified persisting disparity, where Aboriginal and Torres Strait Islander children and young adults had less access to transplant within five years of kidney failure diagnosis, and higher utilisation of deceased donor transplantation, explained by lower living donor or pre‐emptive transplantation. Furthermore, poorer ten‐year survival was observed among Aboriginal and Torres Strait Islander children and young adults who remained on dialysis and could not access transplantation.
When we examined graft survival, we found that Aboriginal and Torres Strait Islander children and young adults graft survival was similar to that for non‐Indigenous children and young adults at five years but was lower at ten years. Similar findings were reported for First Nations adults in Australia18 and overseas.19 Longer cold ischaemia time (particularly longer than 18 hours) was more frequent among Aboriginal and Torres Strait Islander than non‐Indigenous kidney transplant recipients in our study. Protracted cold ischaemia time is a risk factor for delayed graft function, which in turn affects long term graft outcomes.19,20 One reason for longer cold ischaemia time could be distance, as transplantation centres are located in metropolitan areas. In our study, a larger proportion of Aboriginal and Torres Strait Islander children and young adults resided in remote Australia. The impact of prolonged cold ischaemia time could be mitigated by increasing their access to regionally based paediatric transplantation services, proactively solving transportation problems, and using innovative organ preservation methods.21
We also identified higher HLA mismatches for Aboriginal and Torres Strait Islander children and young adults, consistent with findings of higher HLA mismatches and immune‐sensitisation among adult Indigenous kidney transplant recipients.22 HLA mismatches are known to contribute to poorer graft outcomes.23,24 Reasons for higher HLA mismatches among Aboriginal and Torres Strait Islander children and young adults can include lower donation rates, of both living and deceased organs from Aboriginal and Torres Strait Islander communities.25,26 Some identified barriers to organ donation include inadequate transmission of key information to families by health professionals, hesitancy by recipients to request donation given the burden of medical conditions shared by family members, worry about its effects on donors,27,28,29,30 and cultural bias, as documented for adult nephrology care systems.31 Much work is required to improve open communication between health care providers and people with end‐stage kidney disease, including involvement of family members in these conversations, more efficient recipient and donor preparation, and engaging with community preferences for involvement of community elders in improving shared understanding of kidney health and the roles of living and deceased organ donation and organ transplantation.26
The most frequent causes of graft failure for both Aboriginal and Torres Strait Islander and non‐Indigenous children and young adults were chronic allograft nephropathy, acute rejection, and technical or vascular problems. Non‐adherence to post‐transplantation care as the cause of graft loss was more frequently recorded by ANZDATA Registry clinical teams for Aboriginal and Torres Strait Islander children and young adults than for non‐Indigenous recipients (23% v 6.2%). The goal of immune suppression is consistency of optimal dosage to protect grafts from HLA mismatch‐related rejection, while avoiding opportunistic infections. Proactively responding to factors that lead to graft loss and involve medical non‐adherence is consequently crucial for supporting graft and recipient survival,32,33,34 such as minimising the complexity of the medication regime, involving clinical pharmacists, and providing relevant educational and behavioural interventions.35 Other strong recommendations include employing First Nations people in clinical care in transplantation units and in local care, including peer navigators who aid both health staff and families with culturally based understanding of transplantation care,36 and improving family understanding and ability to participate in living organ donation.37
Sovereignty with respect to health outcomes
Our report is based on quantitative data for health outcomes provided by renal services, collated by ANZDATA, which reflect Australian clinician–patient partnerships since 1963. Since intense family and clinical service impact arises from paediatric end‐stage kidney disease, we described the availability of transplantation care for Australian children and young adults over this whole period. Our report therefore provides an indirect, but global, picture of the performance of the Australian health system to build knowledge of when and how models of care contributed to those outcomes. Australian transplant policy already advocates for paediatric priority allocation; however, this study identified persisting inequity, and this requires an urgency for additive priorities for Aboriginal and Torres Strait Islander children. These priorities include preventing the onset of chronic kidney disease after acute kidney injury,38 preventing diabetic nephropathy following young onset diabetes, and when their kidney function reaches end‐stage, prioritising health care models which support their access to dialysis, transplant waitlisting, and achieving early transplantation (especially within five years).39 These recommendations support the National Indigenous Kidney Transplantation Taskforce position statement, which references improving young peoples’ kidney health and wellbeing through transplantation.39
Limitations
One limitation of the source data, which begins in 1963, is the lack of Indigenous Data Governance Policy, which was only implemented in late 2020.17 Indigenous Data Governance which is systemically applied to the Registry now paves a way for data archiving and reporting that references cultural safety and cultural responsibility in data management and data sovereignty. For instance, “non‐adherence to treatment regimens” as described within ANZDATA reflects a clinician perspective on a patient outcome, but this study suggests high value in extending its meaning inclusive of “non‐adherence to treatment regimens due to policy, systems, resourcing, and services‐level inputs”. Strengthening coding of ANZDATA variables is one way that clinical quality registries can meaningfully support audit and review cycles, which holds high value for Aboriginal and Torres Strait Islander families and health carers dedicated to equitably improving paediatric nephrology care outcomes.
Conclusions
Since 1963, we observed excellent five‐year graft and recipient survivals among all children and young adults who achieved transplantation. We note, however, fewer transplants were achieved within the first five years of dialysis initiation among Aboriginal and Torres Strait Islander children and young adults, and their continuing on dialysis without transplant was associated with lower patient survival. Models of care that achieve transplantation within five years of dialysis commencement must be prioritised for Aboriginal and Torres Strait Islander children and young adults.
Box 1 – Characteristics of children and young adults (0–24 years) at the commencement of kidney replacement therapy, Australia, 1963–2020, by group
|
Characteristic |
Aboriginal and Torres Strait Islander |
Non‐Indigenous |
P |
||||||||||||
|
|
|||||||||||||||
|
All patients |
213 (5.8%) |
3523 (94.2%) |
|
||||||||||||
|
Age (years), median (IQR) |
20 (15–22) |
18 (13–22) |
0.003 |
||||||||||||
|
Sex |
|
|
< 0.001 |
||||||||||||
|
Male |
114 (53.5%) |
1412 (40.1%) |
|
||||||||||||
|
Female |
99 (46.5%) |
2111 (59.9%) |
|
||||||||||||
|
Primary kidney disease |
|
|
< 0.001 |
||||||||||||
|
Glomerulonephritis |
97 (46.0%) |
1465 (41.8%) |
|
||||||||||||
|
Congenital malformation of kidney and urinary tract |
55 (26.1%) |
1257 (35.8%) |
|
||||||||||||
|
Polycystic disease |
0 |
65 (1.9%) |
|
||||||||||||
|
Diabetic nephropathy |
14 (6.6%) |
45 (1.3%) |
|
||||||||||||
|
Hypertension |
10 (4.7%) |
39 (1.1%) |
|
||||||||||||
|
Other |
35 (16.4%) |
636 (18.1%) |
|
||||||||||||
|
Other medical conditions |
|
|
|
||||||||||||
|
Diabetes mellitus |
20 (10.2%) |
72 (2.3%) |
< 0.001 |
||||||||||||
|
Chronic lung disease |
14 (7.1%) |
99 (3.2%) |
0.003 |
||||||||||||
|
Coronary artery disease |
6 (3.0%) |
37 (1.2%) |
0.039 |
||||||||||||
|
Cerebrovascular disease |
3 (1.5%) |
29 (0.9%) |
0.40 |
||||||||||||
|
Peripheral vascular disease |
4 (2.0%) |
29 (0.9%) |
0.13 |
||||||||||||
|
Remoteness13 |
|
|
< 0.001 |
||||||||||||
|
Major city |
55 (29.1%) |
1901 (72.7%) |
|
||||||||||||
|
Regional |
63 (33.3%) |
671 (25.6%) |
|
||||||||||||
|
Remote |
71 (37.6%) |
44 (1.7%) |
|
||||||||||||
|
Late referral to nephrologist |
57 (26.8%) |
537 (15.2%) |
< 0.001 |
||||||||||||
|
Era |
|
|
< 0.001 |
||||||||||||
|
1963–1969 |
0 |
96 |
|
||||||||||||
|
1970–1985 |
22 (10.3%) |
862 (25.2%) |
|
||||||||||||
|
1986–1997 |
51 (23.9%) |
881 (25.7%) |
|
||||||||||||
|
1998–2004 |
35 (16.4%) |
476 (13.9%) |
|
||||||||||||
|
2005–2020 |
105 (49.3%) |
1208 (35.2%) |
|
||||||||||||
|
Status at transition |
|
|
< 0.001 |
||||||||||||
|
Dialysis |
127(59.6%) |
960 (27.2%) |
|
||||||||||||
|
Transplant |
58 (27.2%) |
2121 (60.2%) |
|
||||||||||||
|
Death |
27 (12.7%) |
392 (11.1%) |
|
||||||||||||
|
Own kidney function recovered |
1 (0.5%) |
16 (0.5%) |
|
||||||||||||
|
Lost to follow‐up |
0 |
34 (1.0%) |
|
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
Box 2 – Characteristics of children and young adults (0–24 years) at time of kidney transplantation, Australia, 1970–2020, by group
|
Characteristic |
Aboriginal and Torres Strait Islander |
Non‐Indigenous |
P |
||||||||||||
|
|
|||||||||||||||
|
All patients |
93 (3.4%) |
2669 (96.6%) |
|
||||||||||||
|
Age at transplantation (years), median (IQR) |
17 (13–21) |
18 (12–22) |
0.50 |
||||||||||||
|
Sex |
|
|
0.024 |
||||||||||||
|
Female |
45 (48%) |
1603 (60.1%) |
|
||||||||||||
|
Male |
48 (52%) |
1066 (39.9%) |
|
||||||||||||
|
Pre‐emptive transplantation |
1 (1.1%) |
363 (13.6%) |
< 0.001 |
||||||||||||
|
Living donor transplants |
19 (20%) |
1170 (43.9%) |
< 0.001 |
||||||||||||
|
Human leukocyte antigen mismatch |
|
|
< 0.001 |
||||||||||||
|
Two or fewer |
24 (27%) |
944 (43.2%) |
|
||||||||||||
|
Three or four |
26 (32%) |
950 (43.4%) |
|
||||||||||||
|
Five or six |
36 (41%) |
293 (13.4%) |
|
||||||||||||
|
Missing data |
7 |
482 |
|
||||||||||||
|
Panel‐reactive antibodies (PRA) |
|
|
0.001 |
||||||||||||
|
0–49% |
80 (86%) |
2425 (94.2%) |
|
||||||||||||
|
50–100% |
13 (14%) |
149 (5.8%) |
|
||||||||||||
|
Missing data |
0 |
95 |
|
||||||||||||
|
Cold ischaemia time |
|
|
< 0.001 |
||||||||||||
|
Less than 12 hours |
37 (44%) |
1464 (70.4%) |
|
||||||||||||
|
12–18 hours |
22 (26%) |
276 (13.3%) |
|
||||||||||||
|
More than 18 hours |
26 (31%) |
341 (16.6%) |
|
||||||||||||
|
Missing data |
5 |
588 |
|
||||||||||||
|
Donor age (years), median (IQR) |
41 (25–47) |
39 (25–48) |
0.90 |
||||||||||||
|
Transplantation era* |
|
|
0.004 |
||||||||||||
|
1970–1985 |
10 (11%) |
666 (25.0%) |
|
||||||||||||
|
1986–1997 |
27 (29%) |
648 (24.3%) |
|
||||||||||||
|
1998–2004 |
11 (12%) |
369 (13.8%) |
|
||||||||||||
|
2005–2020 |
45 (48%) |
919 (34.4%) |
|
||||||||||||
|
Unknown |
0 |
67 (2.5%) |
|
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Differences by era are described for 1970–2020, as zero Aboriginal and Torres Strait Islander children recorded during 1963–1969. |
|||||||||||||||
Box 3 – Death‐censored kidney graft survival for children and young adults (0–24 years), Australia, 1970–2020, by group: Kaplan–Meier analysis
Box 4 – Causes of graft failure in 1436 children and young adults (0–24 years) after kidney transplantation, Australia, 1970–2020, by group*
|
Cause of graft failure |
Aboriginal and Torres Strait Islander |
Non‐Indigenous |
|||||||||||||
|
|
|||||||||||||||
|
Number of people |
53 |
1383 |
|||||||||||||
|
Acute rejection |
6 (11%) |
226 (16.3%) |
|||||||||||||
|
Chronic allograft nephropathy |
29 (55%) |
800 (57.8%) |
|||||||||||||
|
Hyperacute rejection |
0 |
13 (0.9%) |
|||||||||||||
|
Vascular |
1 (22%) |
71 (5.1%) |
|||||||||||||
|
Technical |
0 |
22 (1.6%) |
|||||||||||||
|
Glomerulonephritis |
2 (4%) |
87 (6.3%) |
|||||||||||||
|
Non‐adherence to therapy |
12 (23%) |
77 (5.6%) |
|||||||||||||
|
Other |
3 (6%) |
87 (6.3%) |
|||||||||||||
|
|
|||||||||||||||
|
* Excludes graft failures without record causes. Median follow‐up time: Aboriginal and Torres Strait Islander children and young adults: 4.5 (interquartile range, 2.6–7.6) years; non‐Indigenous children and young adults: 5.9 (interquartile range, 1.2–12.9) years. |
|||||||||||||||
Box 5 – Survival for children and young adults (0–24 years) after kidney transplantation, Australia, 1970–2020, by group: Kaplan–Meier analysis
Box 6 – Causes of death after kidney transplantation for 822 children and young adults (0–24 years), Australia, 1970–2020, by group*
|
Cause of death |
Aboriginal and Torres Strait Islander |
Non‐Indigenous |
|||||||||||||
|
|
|||||||||||||||
|
Number of people |
28 |
794 |
|||||||||||||
|
Cardiovascular |
13 (46%) |
263 (33.1%) |
|||||||||||||
|
Withdrawal from kidney replacement therapy |
1 (4%) |
70 (8.8%) |
|||||||||||||
|
Cancer |
1 (4%) |
113 (14.2%) |
|||||||||||||
|
Infection |
5 (18%) |
152 (19.1%) |
|||||||||||||
|
Other |
8 (29%) |
196 (24.7%) |
|||||||||||||
|
|
|||||||||||||||
|
* Includes all deaths after kidney transplantation. Median follow‐up time: Aboriginal and Torres Strait Islander children and young adults: 15.0 (interquartile range, 6.9–23.8) years; non‐Indigenous children and young adults: 15.3 (interquartile range, 6.3–26.5) years. |
|||||||||||||||
Box 7 – Survival for children and young adults (0–24 years) on dialysis, Australia, 1963–2020, by group: Kaplan–Meier analysis
Box 8 – Causes of death for 405 children and young adults (0–24 years) who remained on dialysis, Australia, 1963–2020, by group*
|
Cause of death |
Aboriginal and Torres Strait Islander |
Non‐Indigenous |
|||||||||||||
|
|
|||||||||||||||
|
Number of people |
59 |
346 |
|||||||||||||
|
Cardiovascular |
37 (63%) |
144 (41.6%) |
|||||||||||||
|
Withdrawal |
2 (3%) |
35 (10.1%) |
|||||||||||||
|
Cancer |
0 |
32 (9.2%) |
|||||||||||||
|
Infection |
9 (15%) |
58 (16.8%) |
|||||||||||||
|
Other |
11 (19%) |
77 (22.3%) |
|||||||||||||
|
|
|||||||||||||||
|
* Includes all deaths of young people on dialysis. Median follow‐up time: Aboriginal and Torres Strait Islander children and young adults: 4.5 (interquartile range, 1.9–9.6) years; non‐Indigenous children and young adults: 4.9 (interquartile range, 1.4–16.6) years. |
|||||||||||||||
Received 14 January 2024, accepted 8 May 2024
- Swasti Chaturvedi1,2
- Shahid Ullah2
- Jaquelyne T Hughes (Wagadagam)3,4
- 1 Sydney Children's Hospital, Sydney, NSW
- 2 College of Medicine and Public Health, Flinders University, Adelaide, SA
- 3 Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Darwin, NT
- 4 Royal Darwin Hospital, Darwin, NT
Open access:
Open access publishing facilitated by Flinders University, as part of the Wiley ‐ Flinders University agreement via the Council of Australian University Librarians.
Data sharing:
The data reported here have been supplied by the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). The interpretation and reporting of these data are the responsibility of the authors and in no way an official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry. Requests for access to ANZDATA should be directed to ANZDATA.
Jaquelyne Hughes is supported by a National Health and Medical Research Council Emerging Leadership Fellowship (1174758). The authors recognise Indigenous peoples research leadership and their knowledges contained within the following cited references: 8–11, 16, 17, 25, 26, 31, 36, 37 and 39.
No relevant disclosures.
- 1. McCarthy H, Davies C, Au et al.. Paediatric patients with kidney failure requiring replacement therapy. 46th Annual Report Australia and New Zealand Dialysis and Transplant Registry. https://www.anzdata.org.au/wp‐content/uploads/2023/09/ANZDATA_AR‐2022‐23_Chapter‐12_F2.pdf (viewed Jan 2024).
- 2. United States Renal Data System. ESRD among children and adolescents. In: USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2023. https://usrds‐adr.niddk.nih.gov/2023/end‐stage‐renal‐disease/8‐esrd‐among‐children‐and‐adolescents (viewed Jan 2024).
- 3. Bonthuis M, Vidal E, Bjerre A, et al. Ten‐year trends in epidemiology and outcomes of pediatric kidney replacement therapy in Europe: data from the ESPN/ERA‐EDTA Registry. Pediatr Nephrol 2021; 36: 2337‐2348.
- 4. McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association. Long‐term survival of children with end‐stage renal disease. N Engl J Med 2004; 350: 2654‐2662.
- 5. Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation 2020; 104: 1437‐1444.
- 6. Patzer RE, Sayed BA, Kutner N, et al. Racial and ethnic differences in pediatric access to preemptive kidney transplantation in the United States. Am J Transplant 2013; 13: 1769‐1781.
- 7. Tjaden LA, Noordzij M, van Stralen KJ, et al; ESPN/ERA‐EDTA Registry Study Group. Racial disparities in access to and outcomes of kidney transplantation in children, adolescents, and young adults: results from the European Society of Pediatric Nephrology/European Renal Association–European Dialysis and Transplant Association Registry. Am J Kidney Dis 2016; 67: 293‐301.
- 8. Hughes JT, Cundale K, Owen KJ, McDonald SP. Advancing accessible kidney transplantation for Aboriginal and Torres Strait Islander people: the National Indigenous Kidney Transplantation Taskforce. Med J Aust 2023; 219(8 Suppl): S3‐S6. https://www.mja.com.au/journal/2023/219/8/advancing‐accessible‐kidney‐transplantation‐aboriginal‐and‐torres‐strait
- 9. Garrard E, McDonald S. Improving access to and outcomes of kidney transplantation for Aboriginal and Torres Strait Islander people in Australia. Transplant Society of Australia and New Zealand (TSANZ) Performance Report. 2019. https://www.anzdata.org.au/wp‐content/uploads/2019/07/TSANZ‐Performance‐Report‐Improving‐Indigenous‐Transplant‐Outcomes‐Final‐edited‐1.pdf (viewed Jan 2024).
- 10. Kulkarni H, Hughes JT. Worker equity in renal medicine. Intern Med J 2022; 52: 1859‐1862.
- 11. Chaturvedi S, Ullah S, LePage AK, Hughes JT. Rising incidence of end‐stage kidney disease and poorer access to kidney transplant among Australian Aboriginal and Torres Strait Islander children and young adults. Kidney Int Rep 2021; 6: 1704‐1710.
- 12. Grace BS, Kennedy SE, Clayton PA, McDonald SP. Racial disparities in paediatric kidney transplantation. Pediatr Nephrol 2014; 29: 125‐132.
- 13. Australian Bureau of Statistics. Remoteness Areas. Australian Statistical Geography Standard (ASGS), edition 3, 21 Mar 2023. July 2021 – June 2026. https://www.abs.gov.au/statistics/standards/australian‐statistical‐geography‐standard‐asgs‐edition‐3/jul2021‐jun2026/remoteness‐structure/remoteness‐areas (viewed May 2024).
- 14. Therneau T. A package for survival analysis in R. R package version 3.5‐82023. https://CRAN.R‐project.org/package=survival (viewed Dec 2023).
- 15. Kassambara A KM, Biecek P. survminer: drawing survival curves using ggplot. R package version 0.4.92021. https://CRAN.R‐project.org/package=survminer (viewed Dec 2023).
- 16. Huria T, Palmer SC, Pitama S, et al. Consolidated criteria for strengthening reporting of health research involving indigenous peoples: the CONSIDER statement. BMC Med Res Methodol 2019; 19: 173.
- 17. ANZDATA Registry. Guidance on data requests relating to patient ethnicity. 14 Mar 2023. https://www.anzdata.org.au/wp‐content/uploads/2023/03/2.9‐Guidance_on_data_requests_relating_to_patient_ethnicity_v2023.1.pdf (viewed May 2024).
- 18. Rogers NM, Lawton PD, Jose MD. Kidney transplant outcomes in the indigenous population in the Northern Territory of Australia. Transplantation 2006; 82: 882‐886.
- 19. McGuire C, Kannathasan S, Lowe M, Dow T, Bezuhly M. Patient survival following renal transplantation in Indigenous populations: a systematic review. Clin Transplant 2020; 34: e13760.
- 20. Grenda R. Delayed graft function and its management in children. Pediatr Nephrol 2017; 32: 1157‐1167.
- 21. Hosgood SA, Brown RJ, Nicholson ML. Advances in kidney preservation techniques and their application in clinical practice. Transplantation 2021; 105: e202‐e214.
- 22. Rogers NM, Lawton PD, Jose MD. Plasma cell infiltrates and renal allograft outcomes in indigenous and non‐indigenous people of the Northern Territory of Australia. Nephrology (Carlton) 2011; 16: 777‐783.
- 23. Ruck JM, Jackson AM, Massie AB, et al. Temporal changes in the impact of HLA mismatching among pediatric kidney transplant recipients. Transplantation 2019; 103: 1267‐1271.
- 24. Opelz G, Döhler B, Middleton D, Süsal C. HLA matching in pediatric kidney transplantation: HLA poorly matched living donor transplants versus HLA well‐matched deceased donor transplants. Transplantation 2017; 101: 2789‐2792.
- 25. Zheng C, Teixeira‐Pinto A, Hughes JT, et al. Acute rejection, overall graft loss, and infection‐related deaths after kidney transplantation in Indigenous Australians. Kidney Int Rep 2022; 7: 2495‐2504.
- 26. Walker RC, Abel S, Reynolds A, et al. Experiences, perspectives and values of Indigenous peoples regarding kidney transplantation: systematic review and thematic synthesis of qualitative studies. Int J Equity Health 2019; 18: 204.
- 27. Davison SN, Jhangri GS. Knowledge and attitudes of Canadian First Nations people toward organ donation and transplantation: a quantitative and qualitative analysis. Am J Kidney Dis 2014; 64: 781‐789.
- 28. Devitt J, Anderson K, Cunningham J, et al. Difficult conversations: Australian Indigenous patients’ views on kidney transplantation. BMC Nephrol 2017; 18: 310.
- 29. Fahrenwald NL, Stabnow W. Sociocultural perspective on organ and tissue donation among reservation‐dwelling American Indian adults. Ethn Health 2005; 10: 341‐354.
- 30. Jones MA, Cornwall J. “It's hard to ask”: examining the factors influencing decision‐making among end‐stage renal disease patients considering approaching family and friends for a kidney. N Z Med J 2018; 131: 10‐19.
- 31. Hughes JT, Owen KJ, Kelly J, et al. Cultural bias in kidney care and transplantation: review and recommendations to improve kidney care for Aboriginal and Torres Strait Islander people. Med J Aust 2023; 219(8 Suppl): S11‐S14. https://www.mja.com.au/journal/2023/219/8/cultural‐bias‐kidney‐care‐and‐transplantation‐review‐and‐recommendations‐improve
- 32. Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non‐compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant 2004; 4: 1509‐1513.
- 33. Gandolfini I, Palmisano A, Fiaccadori E, et al. Detecting, preventing and treating non‐adherence to immunosuppression after kidney transplantation. Clin Kidney J 2022; 15: 1253‐1274.
- 34. Butler JA, Roderick P, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004; 77: 769‐776.
- 35. Foster BJ, Pai ALH, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE‐IT). Am J Kidney Dis 2018; 72: 30‐41.
- 36. Cundale K, McDonald SP, Irish A, et al. Improving equity in access to kidney transplantation: implementing targeted models of care focused on improving timely access to waitlisting. Med J Aust 2023; 219(8 Suppl): S7‐S10. https://www.mja.com.au/journal/2023/219/8/improving‐equity‐access‐kidney‐transplantation‐implementing‐targeted‐models‐care
- 37. Chaturvedi S, Hughes JT, Cherian S, Morris P. Early evaluation of a newly commenced paediatric nephrology service in the Northern Territory of Australia: a carer's and provider's perspective. J Paediatr Child Health 2020; 56: 1999.
- 38. Chong HYC, Hung TY, Hohls A, et al. Clinical characteristics of hospitalised children with acute post‐streptococcal glomerulonephritis in the Top End of Australia. J Paediatr Child Health 2023; 59: 735‐742.
- 39. National Indigenous Kidney Transplantation Taskforce. Transplantation equity for Aboriginal and Torres Strait Islander Peoples with kidney disease [position statement]. Dec 2022. https://www.niktt.com.au/positionstatement (viewed May 2024).






Abstract
Objectives: To assess differences between Aboriginal and Torres Strait Islander and non‐Indigenous Australian children and young adults in access to and outcomes of kidney transplantation.
Study design: A cohort study based on prospectively collected data; analysis of Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) data.
Setting, participants: Children and young adults aged 0–24 years who commenced kidney replacement therapy in Australia during 1963–2020.
Main outcome measures: Proportions of children and young adults who received kidney transplants within five years of commencing dialysis; 5‐ and 10‐year death‐censored graft survival; and 5‐ and 10‐year survival of children and young adults who received kidney transplants or who remained on dialysis.
Results: During 1963–2020, 3736 children and young adults received kidney replacement therapy in Australia: 213 (5.8%) Aboriginal and Torres Strait Islander and 3523 (94.2%) non‐Indigenous children and young adults. During follow‐up (median, eight years; interquartile range [IQR], 2.6–15 years), 2762 children and young adults received kidney transplants: 93 Aboriginal and Torres Strait Islander (43.7% of those receiving kidney replacement therapy) and 2669 non‐Indigenous children and young adults (75.8%). Smaller proportions of Aboriginal and Torres Strait Islander than of non‐Indigenous children and young adults received transplants within five years of commencing dialysis (99, 46%v 2924, 83.0%), received living donor transplants (19, 20% v 1170, 43.9%), or underwent pre‐emptive transplantation (one, 1.1% v 363, 13.6%). Five‐year graft survival for Aboriginal and Torres Strait Islander recipients was similar to non‐Indigenous recipients (61% v 75%; adjusted hazard ratio [aHR], 1.43; 95% confidence interval [CI], 0.02–2.05), but 10‐year graft survival was lower (35% v 61%; aHR, 1.69; 95% CI, 1.25–2.28). Five‐ and 10‐year survival after kidney transplantation was similar for Aboriginal and Torres Strait Islander and non‐Indigenous people. Among those who remained on dialysis, 10‐year survival was poorer for Aboriginal and Torres Strait Islander than non‐Indigenous children and young adults (aHR, 1.50; 95% CI, 1.08–2.10).
Conclusions: Five‐year graft and recipient survival were excellent for Aboriginal and Torres Strait Islander children and young adults who received kidney transplants; however, a lower proportion received transplants within five years of dialysis initiation, than non‐Indigenous children and young adults. Improving transplant access within five years of dialysis commencement should be a priority.