Opioid agonists, such as methadone and buprenorphine, are effective for treating opioid dependence. In 2022, more than 55 000 Australians were receiving opioid agonist treatment on any day.1 As alternatives to sublingual buprenorphine and buprenorphine–naloxone formulations, two long‐acting depot buprenorphine products, requiring subcutaneous injection once a week or month, were listed by the Pharmaceutical Benefits Scheme (PBS) for subsidised dispensing in October 2019 (Buvidal; Camurus AB2,3) and May 2020 (Sublocade; Indivior4).
Monitoring the uptake of long‐acting depot buprenorphine medications is important for planning treatment services. Estimates of the number of people receiving opioid agonist treatment are derived from collated health department data on individual treatment permits and annual service censuses, reported by the Australian Institute of Health and Welfare in the National Opioid Pharmacotherapy Statistics Annual Data collection.1 However, some states (eg, New South Wales, Queensland) do not distinguish between people receiving different buprenorphine formulations. PBS dispensing data cannot be used for estimating numbers because a large proportion of people receive opioid agonist treatment in services (clinics, hospitals, prisons) where dispensing is not covered by the PBS.
We therefore used opioid medication sales data to estimate the sales‐based patient equivalent (SPE) as a proxy measure of the maximum number of people treated with each buprenorphine formulation. The monthly SPE for sublingual buprenorphine was calculated by dividing the total amount (in milligrams) sold to pharmacies, clinics, hospitals, and prisons by the number of days in each month and the estimated mean daily sublingual buprenorphine dose (based on Australian studies: 14 mg).5 The monthly SPE for long‐acting depot buprenorphine was estimated as the number of monthly injections (each corresponding to one person) and the number of weekly doses sold divided by four. Buprenorphine sales data for 1 September 2019 – 28 February 2023 were provided by the pharmaceutical companies (sublingual buprenorphine: Invidior; depot buprenorphine: Camurus, Invidior). We did not seek formal ethics approval for our analysis of population‐level data that did not allow identification of individual persons.
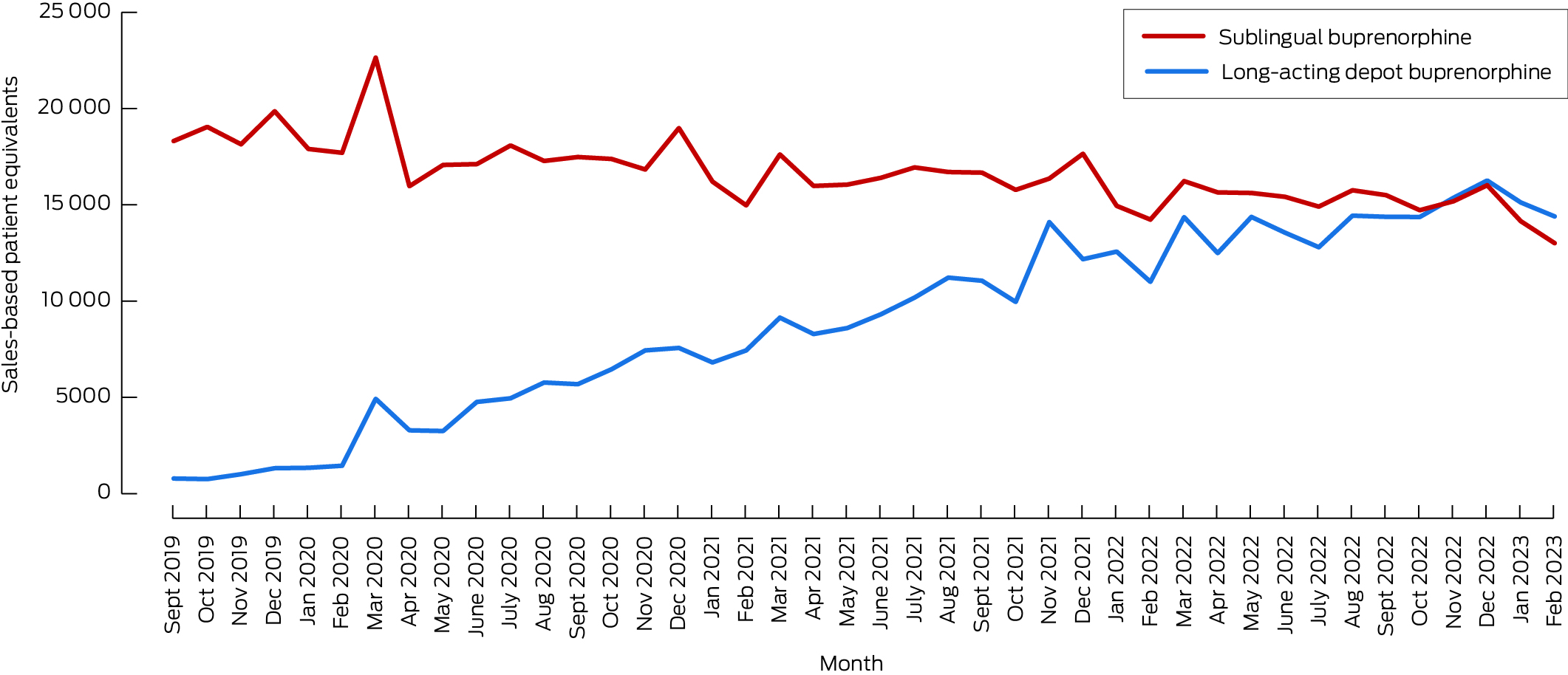
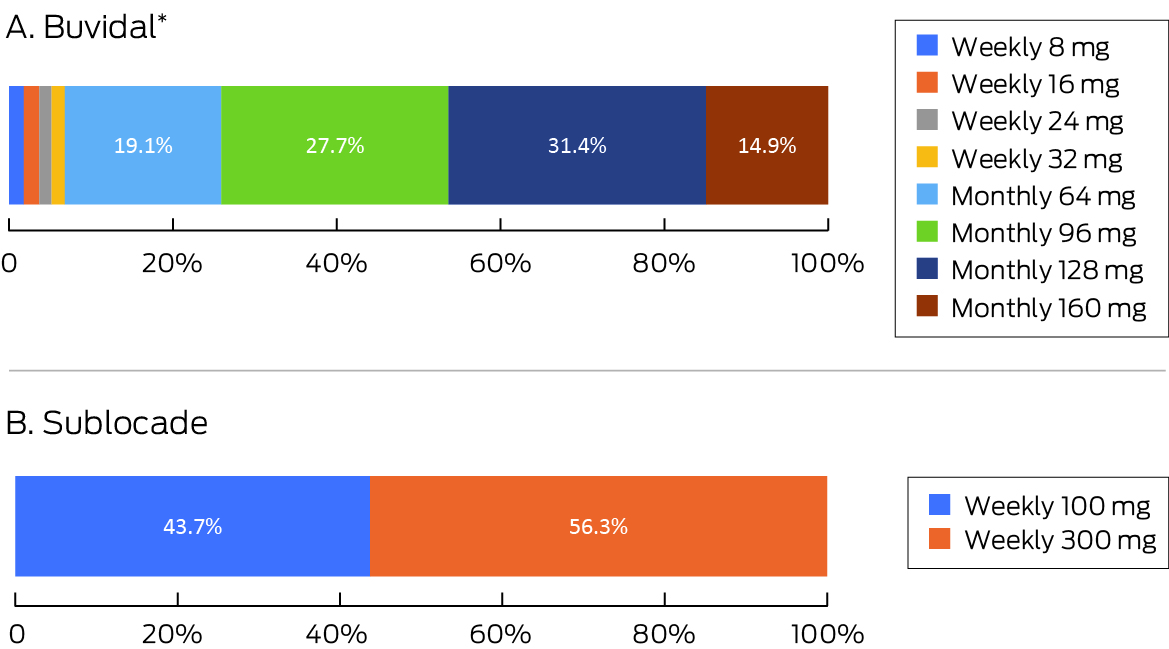
In September–October 2019, an estimated 19 458 people received buprenorphine: 18 686 as sublingual buprenorphine (96.0%) and 772 as depot buprenorphine (4.0%). In December 2022, an estimated 32 287 people received buprenorphine: 16 020 as sublingual buprenorphine (49.6%) and 16 267 as depot buprenorphine (50.4%) (Box 1). In December 2022, 93.1% of people receiving depot Buvidal received monthly rather than weekly injections; 43.7% of those who received Sublocade received 100 mg doses, 56.3% received 300 mg doses (56.3%) (Box 2).
We estimate that half of all people with opioid dependence treated with buprenorphine in Australia now receive it as a long‐acting depot formulation. Reasons for the rapid uptake of this approach during 2019–2022 include strong demand from treated people and the reduced burden of engaging in treatment,5 reduced stigmatisation of people receiving depot buprenorphine,6 changes to treatment in services caused by the coronavirus disease 2019 pandemic,7 and the increased use of long‐acting depot buprenorphine in prisons.8
We estimated the number of people receiving buprenorphine on the basis of sales data, and the estimated mean daily dose for daily sublingual buprenorphine dose was derived from other studies. Limitations to this approach include monthly variations in sales data (reflecting pharmacy and clinic stock levels) and not accounting for medication destroyed locally (eg, expired stock) or not used, which could lead to overestimating the number of people who received buprenorphine.
In conclusion, the use of long‐acting depot buprenorphine increased markedly during 2019–2022, marking a shift from daily opioid agonist treatments.
Box 1 – Estimated number of people who received buprenorphine for treatment of opioid dependence, Australia, 1 September 2019 – 28 February 2023, based on reported sales numbers: by formulation type
Received 9 July 2023, accepted 19 October 2023
- 1. Australian Institute of Health and Welfare. National Opioid Pharmacotherapy Statistics Annual Data Collection. Updated 20 Apr 2023. https://www.aihw.gov.au/reports/alcohol‐other‐drug‐treatment‐services/national‐opioid‐pharmacotherapy‐statistics/data (viewed June 2023).
- 2. Therapeutic Goods Administration. Buvidal monthly (buprenorphine) solution for injection [Australian product information]. 28 Nov 2018. https://www.tga.gov.au/sites/default/files/auspar‐buprenorphine‐191106‐pi‐01.pdf (viewed Oct 2023).
- 3. Therapeutic Goods Administration. Australian Buvidal weekly (buprenorphine) solution for injection [Australian product information]. 28 Nov 2018; revised 30 Aug 2023. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP‐2018‐PI‐02610‐1&d=20230428172310101 (viewed Oct 2023).
- 4. Therapeutic Goods Administration. Sublocade (buprenorphine) [Australian product information]. 18 July 2019; revised 30 Oct 2023. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP‐2019‐PI‐01756‐1&d=20230428172310101&d=20230514172310101 (viewed Oct 2023).
- 5. Lintzeris N, Dunlop AJ, Haber PS, et al. Patient‐reported outcomes of treatment of opioid dependence with weekly and monthly subcutaneous depot vs daily sublingual buprenorphine: a randomized clinical trial. JAMA Netw Open 2021; 4: e219041.
- 6. Barnett A, Savic M, Lintzeris N, et al. Tracing the affordances of long‐acting injectable depot buprenorphine: a qualitative study of patients' experiences in Australia. Drug Alcohol Depend 2021; 227: 108959.
- 7. Lintzeris N, Deacon RM, Hayes V, et al. Opioid agonist treatment and patient outcomes during the COVID‐19 pandemic in south east Sydney, Australia. Drug Alcohol Rev 2022; 41: 1009‐1019.
- 8. Little S, White B, Moensted M, et al. Health and correctional staff acceptability of depot buprenorphine in NSW prisons. Int J Drug Policy 2023; 114: 103978.






Correspondence: nick.lintzeris@sydney.edu.au
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
We acknowledge Ruari MacDonald (Camurus AB) and Jozef Bojkovsky (Indivior) for providing Australian sales data for buprenorphine products.
Nicholas Lintzeris has received funding for unrelated research from Camurus AB and Indivior. Victoria Hayes has received honoraria from Camurus AB for educational sessions at professional development events. Adrian J Dunlop has received grants from Braeburn/Camurus AB for clinical studies using buprenorphine products, and he was an honorary investigator on an Indivior‐funded study of buprenorphine products.