The National Health and Medical Research Council (NHMRC) of Australia is responsible for developing guidelines and evidence statements on a wide range of public and environmental health issues. Since 2015, this has included public health advice on electronic cigarettes (e‐cigarettes). NHMRC issued a Chief Executive Officer (CEO) statement in 2017.1 This article reports on the 2022 update to the NHMRC CEO statement on electronic cigarettes.
“E‐cigarette” is an umbrella term for a range of devices that heat liquid to form an aerosol or vapour. The liquid in e‐cigarettes (e‐liquid) may contain nicotine and includes vegetable glycerine and propylene glycol.2 Flavours and other chemicals are often added to the e‐liquid. The resulting aerosol is quite different from cigarette smoke, with an absence or reduction of some smoke constituents but the addition of other chemicals and metals not associated with cigarette smoke. The device, the liquid mixture used, and variable heating temperatures can influence what is ultimately inhaled by e‐cigarette users.3 E‐cigarette devices have evolved from the early cigarette lookalike products to tank‐based systems, where users add their own nicotine and liquids, to the current disposable and pre‐filled pod products available.4
In Australia, the 2016 National Drug Strategy Household Survey (NDSHS)5 identified that 8.8% of the population aged over 14 years had “ever used” an e‐cigarette.6,7 This increased to 11.3% of the total population aged over 14 years ever having used an e‐cigarette in the 2019 NDSHS survey, and 6.9% of non‐smokers over the age of 14 years reported ever having used an e‐cigarette.7,8 Among those who had tried e‐cigarettes, frequency of use also increased, with more people using them at least monthly (from 10.3% in 2016 to 17.9% in 2019).7,8 Adult daily e‐cigarette use in Victoria increased from 0.9% to 2.4% from 2018–19 to 2022.9
Until early 2020, the majority of e‐cigarette use in Australia was with modifiable tank‐based systems. Since 2020, based on seized products and usage observations from a range of sources, disposable e‐cigarettes now dominate.10 These are relatively low cost for first users, compared with tank systems; include nicotine in high concentration as nicotine salt; and come with colourful flavour descriptions designed to attract target users.11 State‐based data, such as in New South Wales, suggest that this change from tank‐based to disposable high concentration devices has coincided with marked increase in e‐cigarette use by young people.12 Between 2020–21 and 2021–22, daily or occasional e‐cigarette use in NSW by 16–24‐year‐olds increased from 11.1% to 16.5%.12
The combination of evolving product design, publication of new and emerging evidence, and growing concern among public health professionals about the increased use of e‐cigarettes, particularly among young people who do not currently smoke combustible cigarettes, led NHMRC to review the current evidence and provide an updated NHMRC CEO statement on electronic cigarettes on their safety and health impacts.
As noted in the NHMRC Statement, nicotine is well understood to be the major addictive substance in tobacco cigarettes.13 Evidence for addictiveness of nicotine per se was therefore not under review. The NHMRC Statement also notes e‐liquids can contain nicotine, even if labelled “nicotine‐free”.14
The NHMRC Statement is intended to support the health of all Australians, not just current smokers. The Statement assists consumers and policy makers in understanding the current evidence relevant to the marketing and use of e‐cigarettes, their impact on smoking initiation and cessation, uptake among youth, dual use with conventional tobacco products, and their overall implications on individual and population health.
Methods
Overview of the revision process
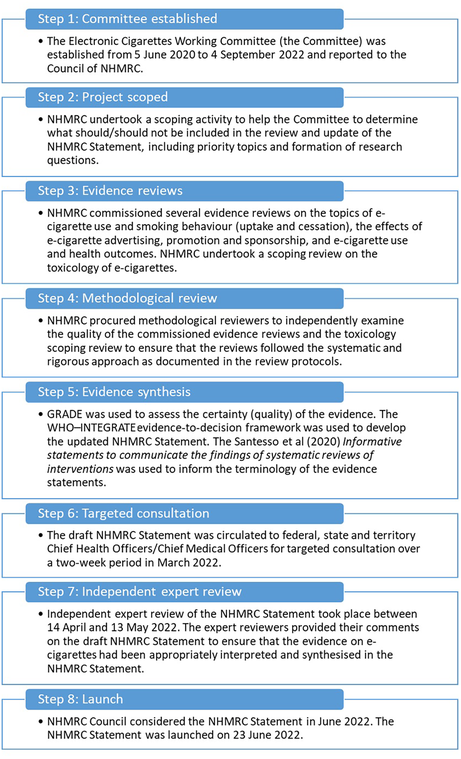
The development of the 2022 NHMRC Statement took two years and followed best practice NHMRC guideline development processes, as outlined in the 2016 NHMRC standards for guidelines15 and the NHMRC Guidelines for guidelines handbook.16 Key stages of the development process are outlined in Box 1. The process drew upon the diverse expertise of the NHMRC Electronic Cigarettes Working Committee17 (established at Step 1) to scope outcomes (Step 2), consider evidence reviews (Step 3), and the independent methodological review of those evidence reviews (Step 4). An evidence‐to‐decision process involved assessment of evidence quality and public health considerations using the WHO–INTEGRATE framework18 (Step 5), targeted consultation (Step 6), and independent review (Step 7) before launch (Step 8).
The Committee considered a comprehensive mix of evidence when updating the NHMRC Statement. This included evidence statements and advice from other countries, such as the United States National Academies of Sciences, Engineering and Medicine19 and Surgeon General reports,20,21 and from Australia, such as the CSIRO (Commonwealth Scientific and Industrial Research Organisation) review22 and the National Industrial Chemicals Notification and Assessment Scheme review.23 This was combined with evidence from commissioned evidence reviews (Step 3). In the evidence‐to‐decision process (Steps 5–7), relevant data on e‐cigarette usage and sociodemographic and other features of the Australian community were also considered.
Populations and outcomes of interest (Step 2)
The Committee first established and determined which populations and outcomes to examine when updating the 2022 NHMRC Statement, in line with the intended use for broad public health. Populations considered were:
- never smokers (those who have never smoked a tobacco cigarette);
- former smokers (those who used to smoke tobacco cigarettes but have quit); and
- current smokers (those who currently smoke tobacco cigarettes).
Detailed outcomes were considered under three headings:
- e‐cigarette use and tobacco smoking uptake and cessation;
- effects of e‐cigarette advertising, promotion and sponsorship; and
- e‐cigarette use and health outcomes.
The Committee did not examine or include evidence on the passive exposure to second hand aerosol from e‐cigarettes or the appeal of e‐cigarettes, including flavours, packaging design and price. The addictiveness of nicotine was not examined as this is well established and widely known.
Evidence reviews (Step 3)
In addition to considering existing international evidence, NHMRC and the Australian Government Department of Health and Aged Care also commissioned specific evidence reviews from external researchers. The Australian Government Department of Health Department of Health and Aged Care commissioned the following evidence reviews:
- E‐cigarette use and combustible tobacco smoking uptake among non‐smokers, including relapse of former smokers: umbrella review, systematic review and meta‐analysis24;
- Efficacy of e‐cigarettes as aids to cessation of combustible tobacco smoking: updated evidence review25; and
- Electronic cigarettes and health outcomes: systematic review of global evidence.26
In addition, NHMRC commissioned the following reviews:
- Effects of e‐cigarette advertising, promotion and sponsorship on people's attitudes, beliefs, perception, intentions and behaviours: a mixed methods systematic review27;
- Supplementary report one: additional material on the review of evidence on the relationship of e‐cigarette use to smoking behaviour, including uptake and cessation28; and
- Supplementary report two: additional material on the review of evidence on the health outcomes of e‐cigarette exposure.29
NHMRC also conducted the following scoping review to be included as part of the evidence base: Inhalation toxicity of non‐nicotine e‐cigarette constituents: risk assessments, scoping review and evidence map (toxicology report).30
Methodological reviews (Step 4)
The research protocols for the reviews commissioned by NHMRC were reviewed by the Committee and independently methodologically reviewed before the reviews commenced. These protocols outlined the scope, research question and methodology.
All evidence reviews, supporting materials and supplementary reports produced were also independently methodologically reviewed to ensure that the methodological quality followed the systematic and rigorous approach documented in the review protocols. The reviews are publicly available from the NHMRC website.13
Evidence synthesis (Step 5)
The internationally recognised Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to assess certainty of the evidence.31 The GRADE methodology aims to improve transparency and consistency in reporting and decision making by assessing key aspects of the way studies are designed, run and analysed, which affects how certain (or confident) reviewers and committees can be that the results reported in studies are accurate. GRADE certainty ratings for the type of epidemiological evidence typical of broad public health exposures are generally low to very low. More highly rated study designs that are considered necessary for clinical practice guidelines, such as randomised controlled trials, are not appropriate for most public health exposures. This is chiefly because randomisation to many public health exposures of interest, such as smoking, alcohol, or early age at first pregnancy, is often not feasible or ethical. To acknowledge that well designed observational studies are often the best source of evidence on public health issues, Committee members agreed that bodies of evidence that consist of randomised controlled trials would assume an initial level of certainty of “high”, prospective cohort studies would assume an initial level of certainty of “moderate”, and all other observational studies would assume an initial level of certainty of “low”. The certainty of the evidence included in the evidence reviews varied across outcomes from moderate (for outcomes relating to the influence of the media on intention to use e‐cigarettes in young people) to low or even very low for many of the other outcomes.
The available evidence had several limitations. E‐cigarettes are relatively new products and the design and technology behind the devices and components used in e‐cigarettes are constantly evolving. The wide variation of e‐cigarettes, including manufacturing quality, setting customisation and variation in e‐liquids used, makes it difficult to ascertain their safety and health impacts. Most of the direct evidence of safety and health impacts of e‐cigarettes was limited to short term human epidemiological or clinical studies, with long term effects mostly unknown.
Most studies examining the toxicological constituents of e‐liquids were in vitro or animal‐based studies.30 These study designs have inherent limitations and are generally considered to provide less direct evidence when compared with human randomised controlled trials or long term epidemiological studies. However, in vitro or animal‐based studies are useful when needing to understand the effects of an environmental exposure to a substance or when it is not ethical or possible to conduct human studies due to the toxic nature of the substances being examined or to control for confounding factors present in human populations.32,33
The evidence on the impact of e‐cigarette marketing on related behaviours, such as intention to use e‐cigarettes or uptake/initiation, and on the impact of e‐cigarette use on smoking uptake/initiation was mainly limited to international cross‐sectional or cohort studies. Very few studies were conducted in Australia.27 Contrastingly, most of the evidence examining the efficacy of e‐cigarettes as a tobacco cigarette smoking cessation tool was from randomised controlled trials based on clinical settings with behavioural support, which may not be generalisable to real‐world effectiveness of e‐cigarettes in settings less supportive of cessation; these studies also had small sample sizes and short follow‐up periods.25
The WHO–INTEGRATE evidence‐to‐decision framework18 is designed for decision making at population levels and was used to develop the 2022 NHMRC Statement. The WHO–INTEGRATE framework was used to shape and write the 2022 NHMRC Statement, including what evidence was included and emphasised in the key messages.34
Targeted consultation (Step 6) and independent expert review (Step 7)
Consultation is a core component of the NHMRC's guideline development process, contributing to accountability of the agency and independence of the advice. The draft 2022 NHMRC Statement and infographics underwent targeted consultation with federal, state and territory Chief Health/Medical Officers over a two‐week period in March 2022. Chief Health/Medical Officers were also asked to contribute Poisons Information Centre data on nicotine poisoning for inclusion in the Statement (if available) and to comment on two infographic concepts that summarised the Statement. Overall, Chief Health/Medical Officers were supportive of the Statement. In response to feedback, edits to the Statement were made, including the addition of a plain English summary.
After revisions in response to targeted consultation, the next version of the 2022 NHMRC Statement and associated resources underwent review by three independent experts to ensure the evidence on e‐cigarettes was appropriately interpreted and synthesised. No errors of fact or significant omissions of evidence were identified. However, the phrasing of the 2022 NHMRC Statement and accompanying resources was clarified at several points. The final Statement and associated resources were released in June 2022 following consideration by the NHMRC Council.
Comparison with international guidance
The 2022 NHMRC Statement is broadly consistent with reports from a number of international health bodies published from 2020 onwards (Box 2). A significant factor contributing to variations between these statements and advice is the way that the questions have been framed,22 with some having a major focus on benefits associated with smoking cessation and others considering a broader range of issues including longer term harms and risks associated with use for purposes other than smoking cessation. The 2022 NHMRC Statement does not compare harms of e‐cigarette use with smoking tobacco cigarettes, reflecting the purpose to support the health of all Australians and to avoid normalisation of nicotine. Relative harm from e‐cigarette use compared with tobacco cigarettes is also difficult to quantify and varies depending on a number of factors such as conditions of use, e‐cigarette device type and setting, e‐liquid type and concentration, and frequency of e‐cigarette and tobacco cigarette use.3,40 The absolute risks of e‐cigarettes cannot be determined at the present time and longer term effects, of importance for young people who become dependent, remain unclear.19 As such, the 2022 NHMRC Statement can be characterised as supporting a continuation of the precautionary approach.
Key messages
Following this extensive evidence synthesis, targeted consultation and expert review, a precautionary approach to e‐cigarettes, particularly for people who have never smoked, remains appropriate. The key messages in the 2022 NHMRC Statement13 include:
- E‐cigarettes can be harmful; all e‐cigarette users are exposed to chemicals and toxins that have the potential to cause adverse health effects.
- E‐cigarette‐related poisonings have substantially increased over the past five years. E‐cigarette‐related calls to Australian Poisons Information Centres have more than doubled between 2020 and 2021.
- There are no health benefits of using e‐cigarettes if you do not currently smoke tobacco cigarettes.
- People who have never smoked may be more likely to take up tobacco smoking if they use e‐cigarettes.
- Adolescents are more likely to try e‐cigarettes if they are exposed to e‐cigarettes on social media.
- Short term e‐cigarette use may benefit smokers if they are able to quit smoking and have been previously unsuccessful with other smoking cessation aids.
- There are other proven safe and effective options available to help smokers quit.
Following the development and publication of the NHMRC Statement, a media release and associated social media content (Box 3) promoting and explaining the Statement were issued. The 2022 NHMRC Statement is an essential synthesis of the current evidence that can now be used by both state and federal health departments in guiding e‐cigarette regulations. Further research is needed in many areas, including on the longer term impacts of e‐cigarette use on the health of young people.
The NHMRC 2022 CEO statement on electronic cigarettes and supporting literature are available at www.nhmrc.gov.au/ecigs.
Box 1 – Overview of the development process of the 2022 National Health and Medical Research Council (NHMRC) statement on electronic cigarettes (e‐cigarettes)
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. Source: Figure reproduced with permission from the National Health and Medical Research Council.13
Box 2 – Snapshot summary comparison of international guidance on electronic cigarettes (e‐cigarettes)
|
Author |
Guidance |
Key messages |
|||||||||||||
|
|
|||||||||||||||
|
Belgian Superior Health Council |
Report 9549: Electronic cigarette: evolution (2022)35 |
|
|||||||||||||
|
World Health Organization |
Tobacco: e‐cigarettes (2022)2 |
|
|||||||||||||
|
Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) |
SCHEER scientific opinion on electronic cigarettes (2021)36 |
|
|||||||||||||
|
United States Preventive Services Task Force |
Interventions for tobacco smoking cessation in adults, including pregnant persons (2021)37 |
|
|||||||||||||
|
Irish Health Research Board |
Electronic cigarette use and tobacco cigarette smoking initiation in adolescents: an evidence review (2020)38 |
|
|||||||||||||
|
Electronic cigarette and smoking cessation: an evidence review (2020)39 |
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – Summary infographic of the National Health and Medical Research Council 2022 CEO statement on electronic cigarettes (e‐cigarettes)

Source: Figure reproduced with permission from the National Health and Medical Research Council.13
Provenance: Not commissioned; externally peer reviewed.
- Becky Freeman1
- Matthew J Peters2,3
- Renee Bittoun4,5
- Richard Brightwell6
- Dallas R English7
- David P Thomas8
- Margaret FA Otlowski9
- Nicholas A Zwar10
- Catherine Chamberlain11,12
- 1 University of Sydney, Sydney, NSW
- 2 Macquarie University, Sydney, NSW
- 3 Concord Repatriation General Hospital, Sydney, NSW
- 4 Avondale University, Cooranbong, NSW
- 5 University of Notre Dame Australia, Sydney, NSW
- 6 Edith Cowan University, Perth, WA
- 7 Centre for MEGA Epidemiology, University of Melbourne, Melbourne, VIC
- 8 Menzies School of Health Research, Charles Darwin University, Darwin, NT
- 9 University of Tasmania, Hobart, TAS
- 10 Bond University, Gold Coast, QLD
- 11 University of Melbourne, Melbourne, VIC
- 12 Judith Lumley Centre, La Trobe University, Melbourne, VIC
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
We would like to show our gratitude and pay our respects to our colleague, Dr Kerry Nugent, who died on 29 December 2022 after a brief illness. Dr Nugent was a highly valued member of the Electronic Cigarettes Working Committee. We are especially grateful for his contributions to the development of the National Health and Medical Research Council (NHMRC)’s 2022 CEO statement on electronic cigarettes, including his sound advice and knowledge on the toxicological aspects of e‐liquids and e‐cigarettes. We also acknowledge the contribution of Jennifer Savenake, Director (Public Health), and the NHMRC project team throughout the development of the NHMRC 2022 CEO statement on electronic cigarettes.
The authors were reimbursed for their time to attend NHMRC Electronic Cigarettes Working Committee meetings. All committee member declarations can be found in full here: https://www.nhmrc.gov.au/about‐us/leadership‐and‐governance/committees/electronic‐cigarettes‐working‐committee.
- 1. National Health and Medical Research Council. CEO statement on electronic cigarettes (e‐cigarettes). Canberra: NHMRC, 2017. https://www.nhmrc.gov.au/sites/default/files/documents/attachments/statement‐electronic‐cigarettes.pdf (Viewed Oct 2023).
- 2. World Health Organization. Tobacco: e‐cigarettes. Geneva: WHO, 2022. https://www.who.int/news‐room/questions‐and‐answers/item/tobacco‐e‐cigarettes#:~:text=Electronic%20cigarettes%20(or%20e%2Dcigarettes,of%20nicotine%20and%20harmful%20emissions (viewed Feb 2023).
- 3. World Health Organization. Electronic nicotine delivery systems and electronic non‐nicotine delivery systems (ENDS/ENNDS). Geneva: WHO, 2016. https://www.who.int/publications/m/item/electronic‐nicotine‐delivery‐systems‐and‐electronic‐non‐nicotine‐delivery‐systems‐(ends‐ennds) (viewed Feb 2023).
- 4. Centers for Disease Control and Prevention. About electronic cigarettes (e‐cigarettes). Atlanta (GA): Office on Smoking and Health, 2023. https://www.cdc.gov/tobacco/basic_information/e‐cigarettes/about‐e‐cigarettes.html (viewed Feb 2023).
- 5. Australian Institute of Health and Welfare. National Drug Strategy Household Survey, 2016 [supplementary data tables: chapter 3, Tobacco; table 3.16: Lifetime use of electronic cigarettes (e‐cigarettes), by age and smoker status, people aged 12 years or older, 2013 and 2016 (per cent)]. Canberra: AIHW, 2016. https://www.aihw.gov.au/getmedia/c679f184‐ca65‐4fc6‐9a00‐dbb4feb56c02/chapter‐3‐tobacco.xlsx.aspx (viewed Oct 2023).
- 6. Tobacco in Australia. Prevalence of e‐cigarette use [table 18.3.1]. Melbourne: Cancer Council, 2019. https://www.tobaccoinaustralia.org.au/chapter‐18‐e‐cigarettes/18‐3‐extent#_ENREF_5 (viewed Oct 2023).
- 7. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2019 [Cat. No. PHE 270]. Canberra: AIHW, 2020. https://www.aihw.gov.au/reports/illicit‐use‐of‐drugs/national‐drug‐strategy‐household‐survey‐2019/contents/summary (viewed Feb 2023).
- 8. Australian Institute of Health and Welfare. National Drug Strategy Household Survey, 2019 [data tables: National Drug Strategy Household Survey 2019: 2 Tobacco smoking, supplementary tables. Table 2.19: Lifetime use of electronic cigarettes (e‐cigarettes), by age and smoker status, 2016 to 2019 (per cent)]. Canberra: AIHW, 2020. https://www.aihw.gov.au/getmedia/e83fc585‐87e9‐466b‐8f63‐6821a74b5528/aihw‐phe‐260‐2‐Tobacco‐smoking‐tables.xlsx.aspx (viewed Oct 2023).
- 9. Bayly M, Mitsopoulos E, Durkin S, Scollo M. E‐cigarette use and purchasing behaviour among Victorian adults: findings from the 2018–19 and 2022 Victorian Smoking and Health Surveys [prepared for Quit Victoria]. Melbourne: Centre for Behavioural Research in Cancer, Cancer Council Victoria; 2022. https://www.cancervic.org.au/downloads/cbrc/R22_MB_E‐cigarette%20use%20and%20purchasing%20behaviour%20among%20Victorian%20adults.pdf (viewed Oct 2023).
- 10. Watts C, Egger S, Dessaix A, et al. Vaping product access and use among 14–17‐year‐olds in New South Wales: a cross‐sectional study. Aust N Z J Public Health 2022; 46: 814‐820.
- 11. NSW Health. The facts about vaping. Sydney: NSW Government, 2022 https://www.health.nsw.gov.au/tobacco/Factsheets/vaping‐teachers‐types.pdf (viewed Feb 2023).
- 12. HealthStats NSW. E‐cigarette use by electronic cigarette use and age (years) [NSW Population Health Survey (SAPHaRI), Centre for Epidemiology and Evidence, NSW Ministry of Health]. Sydney: NSW Health, 2022 https://www.healthstats.nsw.gov.au/#/indicator?name=‐beh‐smo‐ecig‐phs&location=NSW&view=Trend&measure=prevalence&groups=Age%20(years),Electronic%20cigarette%20use&compare=Electronic%20cigarette%20use,Age%20(years)&filter=Electronic%20cigarette%20use,Current%20user,Ever%20used&filter=Age%20(years),All%20ages,16‐24%20years (viewed Feb 2023).
- 13. National Health and Medical Research Council. CEO statement on electronic cigarettes. Canberra: NHMRC, 2022. https://www.nhmrc.gov.au/health‐advice/all‐topics/electronic‐cigarettes/ceo‐statement (viewed Feb 2023).
- 14. Chivers E, Janka M, Franklin P, et al. Nicotine and other potentially harmful compounds in “nicotine‐free” e‐cigarette liquids in Australia. Med J Aust 2019; 210: 127‐128. https://www.mja.com.au/journal/2019/210/3/nicotine‐and‐other‐potentially‐harmful‐compounds‐nicotine‐free‐e‐cigarette
- 15. National Health and Medical Research Council. 2016 NHMRC standards for guidelines. Canberra: NHMRC, 2016. https://www.nhmrc.gov.au/guidelinesforguidelines/standards (viewed Feb 2023).
- 16. National Health and Medical Research Council. Guidelines for guidelines handbook. Canberra: NHMRC, 2016 https://www.nhmrc.gov.au/guidelinesforguidelines (viewed Feb 2023).
- 17. National Health and Medical Research Council. Electronic Cigarettes Working Committee. Canberra: NHMRC, 2022 https://www.nhmrc.gov.au/about‐us/leadership‐and‐governance/committees/electronic‐cigarettes‐working‐committee (viewed Feb 2023).
- 18. Rehfuess EA, Stratil JM, Scheel IB, et al. The WHO–INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective. BMJ Global Health 2019; 4 (Suppl): e000844.
- 19. National Academies of Sciences, Engineering and Medicine. Public health consequences of e‐cigarettes. Washington, DC: National Academy Press, 2018. https://nap.nationalacademies.org/catalog/24952/public‐health‐consequences‐of‐e‐cigarettes (viewed Sept 2023).
- 20. US Department of Health and Human Services. E‐cigarette use among youth and young adults: a report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. https://www.cdc.gov/tobacco/data_statistics/sgr/e‐cigarettes/pdfs/2016_sgr_entire_report_508.pdf (viewed Feb 2023).
- 21. US Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. https://www.hhs.gov/sites/default/files/2020‐cessation‐sgr‐full‐report.pdf (viewed Feb 2023).
- 22. Byrne S, Brindal E, Williams G, et al. E‐cigarettes, smoking and health: a literature review update. CSIRO, 2018. https://www.csiro.au/‐/media/BF/Files/E‐cigarettes/E‐cigarettes‐Consolidated‐Final‐Report240618‐pdf.pdf (viewed Sept 2023).
- 23. Australian Government Department of Health, National Industrial Chemicals Notification and Assessment Scheme. Non‐nicotine liquids for e‐cigarette devices in Australia: chemistry and health concerns. Canberra: Commonwealth of Australia, 2019. https://www.industrialchemicals.gov.au/sites/default/files/2020‐08/Non‐nicotine%20liquids%20for%20e‐cigarette%20devices%20in%20Australia%20chemistry%20and%20health%20concerns%20%5BPDF%201.21%20MB%5D.pdf (viewed Feb 2023).
- 24. Baenziger ON, Ford L, Yazidjoglou A, Joshy G, Banks E. E‐cigarette use and combustible tobacco cigarette smoking uptake among non‐smokers, including relapse in former smokers: umbrella review, systematic review and meta‐analysis. BMJ Open 2021; 11: e045603.
- 25. Yazidjoglou A, Ford L, Baenziger O, et al. Efficacy of e‐cigarettes as aids to cessation of combustible tobacco smoking: updated evidence review — final report prepared for the Australian Government Department of Health. Canberra: National Centre for Epidemiology and Population Health, Australian National University; 2021. https://openresearch‐repository.anu.edu.au/handle/1885/247864 (viewed Feb 2023).
- 26. Banks E, Yazidjoglou A, Brown S, et al. Electronic cigarettes and health outcomes: systematic review of global evidence — report to the Australian Department of Health. Canberra: National Centre for Epidemiology and Population Health, Australian National University; 2022. https://openresearch‐repository.anu.edu.au/bitstream/1885/262914/1/Electronic%20cigarettes%20health%20outcomes%20review_2022_WCAG.pdf (viewed Feb 2023).
- 27. Moola S, Tyagi J, Miller M, et al. Effects of e‐cigarette advertising, promotion, and sponsorship on people's attitudes, beliefs, perceptions, intentions, and behaviours: a mixed methods systematic review — evidence evaluation report. Sydney: George Institute for Global Health, 2021. https://www.nhmrc.gov.au/file/18976/download?token=PMTRLJaL (viewed Feb 2023).
- 28. Banks E, Beckwith K, Yazidjoglou A, et al. Supplementary report one: additional material on the review of evidence on the relationship of e‐cigarette use to smoking behaviour, including uptake and cessation — final report prepared for the National Health and Medical Research Council. Canberra: National Centre for Epidemiology and Population Health, Australian National University; 2021. https://www.nhmrc.gov.au/file/18290/download?token=_CnGFuna (viewed Feb 2023).
- 29. Banks E, Yazidjoglou A, Beckwith K, et al. Supplementary report two: additional material on the review of evidence on the health outcomes of e‐cigarette exposure. Canberra: National Centre for Epidemiology and Population Health, Australian National University; 2021. https://www.nhmrc.gov.au/file/18288/download?token=ILAY7j1l (viewed Feb 2023).
- 30. National Health and Medical Research Council. Inhalation toxicity of non‐nicotine e‐cigarette constituents: risk assessments, scoping review and evidence map. Canberra: NHMRC, 2022. https://www.nhmrc.gov.au/sites/default/files/documents/attachments/ecigarettes/Scoping_review_on_the_inhalation_toxicity_of_non‐nicotine_e‐cigarette_constituents.pdf (viewed Feb 2023).
- 31. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Working Group, 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.9rdbelsnu4iy (viewed Sept 2023).
- 32. Woodruff TJ, Sutton P, Navigation Guide Work Group. An evidence‐based medicine methodology to bridge the gap between clinical and environmental health sciences. Health Aff (Millwood) 2011; 30: 931‐937.
- 33. Woodruff TJ, Sutton P. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect 2014; 122: 1007‐1014.
- 34. Santesso N, Glenton C, Dahm P, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020; 119: 126‐135.
- 35. Conseil Supérieur de la Santé. [Electronic cigarette: evolution] [French]. Brussels: CSS, 2022. https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20220616_css‐9549_avis_e‐cigarette_vweb_0.pdf (viewed Sept 2023).
- 36. Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). SCHEER scientific opinion on electronic cigarettes. Brussels: European Commission, 2021. https://health.ec.europa.eu/system/files/2022‐08/scheer_o_017.pdf (viewed Sept 2023).
- 37. US Preventive Services Task Force; Krist AH, Davidson KW, Mangione CM, et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 2021; 325: 265‐279.
- 38. O'Brien D, Long J, Lee C, et al. Electronic cigarette use and tobacco cigarette smoking initiation in adolescents: an evidence review. Dublin: Health Research Board, 2020. https://www.hrb.ie/fileadmin/2._Plugin_related_files/Publications/2020_publication‐related_files/2020_HIE/Evidence_Centre/Electronic_cigarette_use_and_tobacco_cigarette_smoking_initiation_in_adolescents.pdf (viewed Sept 2023).
- 39. Quigley J, Kennelly H, Lee C, et al. Electronic cigarettes and smoking cessation: an evidence review. Dublin: Health Research Board, 2020. https://www.hrb.ie/fileadmin/2._Plugin_related_files/Publications/2020_publication‐related_files/2020_HIE/Evidence_Centre/Electronic_cigarettes_and_smoking_cessation_systematic_evidence_review.pdf (viewed Sept 2023).
- 40. Lindson‐Hawley N, Hartmann‐Boyce J, Fanshawe TR, et al. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev 2016; (10): CD005231.






Abstract
Introduction: Electronic cigarette (e‐cigarette) use in Australia has rapidly increased since the 2017 National Health and Medical Research Council (NHMRC)Chief Executive Officer (CEO) statement on e‐cigarettes . The type of products available and the demographic characteristics of people using these products have changed. New evidence has been published and there is growing concern among public health professionals about the increased use, particularly among young people who do not currently smoke combustible cigarettes. The combination of these issues led NHMRC to review the current evidence and provide an updated statement on e‐cigarettes. In this article, we describe the comprehensive process used to review the evidence and develop the 2022 NHMRC CEO statement on electronic cigarettes.
Main recommendations:
Changes in management as a result of this statement: The evidence base for the harms of e‐cigarette use has strengthened since the previous NHMRC statement. Significant gaps in the evidence base remain, especially about the longer term health harms of using e‐cigarettes and the toxicity of many chemicals in e‐cigarettes inhaled as an aerosol.