The known: Japanese encephalitis virus (JEV) was first detected in New South Wales in February 2022. As few infections lead to clinical disease, estimating the number of infections in humans is difficult.
The new: One in eleven serosurvey participants, drawn from five regional NSW towns deemed to be at risk on ecological grounds, were seropositive for JEV‐specific antibody, presumably reflecting non‐clinical JEV during the preceding arbovirus season.
The implications: Vaccination against JEV in areas of NSW at particular risk of JEV infection and reducing personal exposure to potentially JEV‐transmitting mosquitoes are essential for reducing the risk of Japanese encephalitis in NSW.
The Japanese encephalitis virus (JEV) is a flavivirus that circulates in 24 countries across the South‐East Asia and Western Pacific regions.1 Many countries in which the virus is endemic have childhood vaccination programs, and older people are often immune following earlier infections.1,2 In Australia, JEV vaccines have been only recommended for people living in the outer Torres Strait islands,3 some laboratory workers, and for people travelling for at least a month in regions where the virus is endemic.4 Consequently, most people in Australia have not been vaccinated against JEV.
An outbreak of Japanese encephalitis was reported in tropical far north Queensland in the 1990s; in February 2021, a case was identified on the Northern Territory tropical coast, but without evidence of broader viral transmission.5 JEV was first detected in temperate southeast Australia in February 2022, in piggeries in New South Wales;6 infections in humans were first reported in March 2022, also in NSW.7 Between 1 January 2021 and 30 June 2022, 42 cases of Japanese encephalitis were reported in five Australian states and territories,8 during a multi‐year La Niña event that caused heavy rainfall and widespread flooding in Australia.9
Epidemics and sporadic infections with other encephalitic flaviviruses, such as Murray Valley encephalitis virus and West Nile clade Kunjin virus, have also been reported in NSW.10 Given the availability of suitable virus vectors, reservoirs, and transmission cycles in NSW, understanding JEV disease epidemiology and planning a vaccination program for early control is critical. We undertook a serosurvey to determine the proportion of people in towns at risk of exposure to the virus with antibodies to JEV, and risk factors for infection.
Methods
We undertook a cross‐sectional serosurvey in NSW. Our data were later included in a national cross‐sectional study of the seroprevalence of JEV‐specific antibodies in Australia.11 We report our study in accordance with the STROBE statement on cross‐sectional studies.12
The serosurvey was conducted in five regional NSW towns during 21 June – 22 July 2022: Balranald, Corowa, Dubbo, Griffith, and Temora, all deemed to be at high risk of JEV infections during January–March 2022 (Box 1). Risk factors for towns included notified cases of Japanese encephalitis during the 2022 outbreak; piggeries with infected animals (within 5 km); mosquito traps or sentinel chicken flocks in which JEV had been detected; and floodplain wetlands where water bird populations may nest (within 5 km).
We invited people (all ages) who lived or worked within 20 km of each town to participate in the survey (convenience sampling). We distributed flyers at health and local community centres and promoted the survey in local media and in social media networks. In larger towns, further recruitment activities were conducted through local businesses, at local government workplaces, and in shopping centres. In each town, an NSW Health or community building was identified as the testing centre. Appointments were not required, and people could attend the testing centre throughout extended business hours over three to five days.
Data for participants were later excluded if the person was born in a country in which JEV is endemic, they had been vaccinated against JEV, or if they had travelled or lived for more than one month in a country where JEV is endemic (China, Vietnam, Philippines, India, Indonesia, Nepal, Sri Lanka, Thailand, Taiwan, Papua New Guinea).
Questionnaire development
The electronic consent form and questionnaire were developed in REDCap 12.0.2. We collected demographic data, information about Japanese encephalitis vaccination or residence in a country where JEV is endemic, and information about risk exposures at home, work, school, or during recreation activities (Supporting Information, part 1). The questionnaire was administered by trained health staff at each site. Each participant was given a unique identifier that was recorded on the questionnaire and the blood collection form to facilitate subsequent linkage. Paper‐based questionnaires were available if requested by participants, and the responses entered by the investigators into REDCap.
Blood collection and serological testing
Whole blood samples (5 mL) were collected by trained health staff via venepuncture, transferred to BD vacutainers, stored at 4°C, and transported according to routine NSW Pathology procedures to the Institute for Clinical Pathology and Medical Research (Westmead Hospital, Sydney) for testing.
We measured JEV‐specific antibody levels in sera using a defined epitope‐blocking enzyme‐linked immunosorbent assay (DEB‐ELISA). The ELISA was prepared in‐house, using purified whole JEV virions as the antigen. After 60 minutes' incubation at 37°C, a JEV‐specific monoclonal antibody conjugate was added. Results were determined semi‐quantitatively as proportional inhibition compared with a negative control; samples for which inhibition was 40% or greater were deemed to be JEV‐specific total antibody‐positive. To differentiate between recent infection and older infections or vaccine‐induced immunity, we assayed IgM in total antibody‐positive samples in an immunofluorescence assay.
Serologic cross‐reactivity can complicate the interpretation of antibody results in areas where multiple flaviviruses circulate. To overcome this problem, DEB‐ELISAs facilitate the detection of epitope‐specific antibody. As the detector antibody, we selected a JEV‐specific monoclonal antibody against a JEV‐specific epitope in the envelope protein; it did not bind the Murray Valley encephalitis, Kunjin, West Nile, yellow fever, dengue (serotypes 1–4), Kokobera, Stratford, Alfuy, or Edge Hill flaviviruses. The sensitivity of the assay was 98.5%, the specificity 100%.
Data analysis
All analyses were conducted in Stata 14.0. We summarise the characteristics of the participants as descriptive statistics, including age, sex, Indigenous status, and township. We assessed the representativeness of our sample by comparing these characteristics with those of the source population, obtained from 2016 Australian Bureau of Statistics NSW mesh block data and extracted for 20 km radii from each town centre in ArcMap 10.6.1.15
Weighted estimates were calculated using cell‐based weighting from census‐derived reference population data.15 Weighted estimates were calculated for the ith stratum as wi × pi × 100, where wi is the population proportion/sample proportion, and pi is the p seropositive results of n total tests. Univariate prevalence odds ratios (PORs) with confidence intervals (CIs) were calculated for exposure risks and protective behaviour using the Stata cctable command. POR was preferred to risk ratios because the assumed prevalence of disease was low.
Ethics approval
The study was initiated as part of an investigation into a public health threat under the NSW Public Health Act 2010. The Sydney Children's Hospital Network Human Research Ethics Committee subsequently approved the study (2022/ETH01167).
Results
Blood samples were collected from 1048 of 1066 people who consented to participation (98.3%); 131 (12.5%) were subsequently excluded from our analysis (country of birth, 48; JEV vaccination, 18; travel in a country in which JEV is endemic, 65). Of the 917 participants included in the analysis, 559 were female (61.0%); the median age was 52 years (interquartile range [IQR], 37–62 years), and 27 (2.9%) were under 20 years of age (source population: 26.1%). Forty‐two were Aboriginal or Torres Strait Islander people (4.6%; source population: 9.9%). Most participants were residents of Griffith (308, 33.6%) or Dubbo (260, 28.4%), reflecting their larger populations (Box 2).
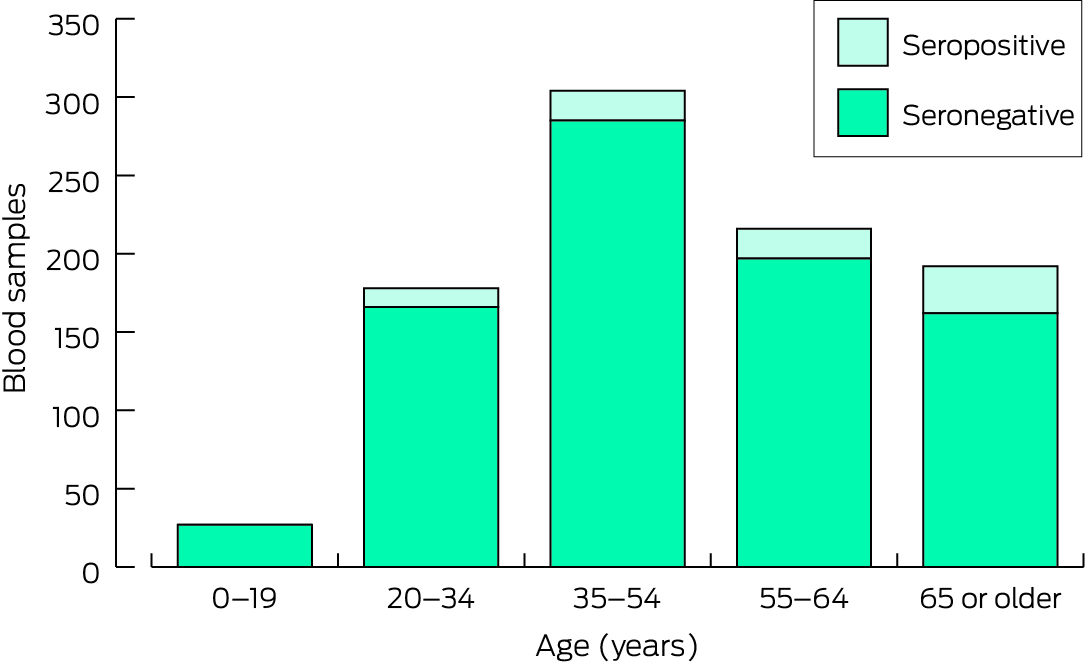
Eighty of 917 participants were seropositive for JEV total antibody (8.7%), including people from each of the five towns (none were IgM‐positive); their ages ranged between 20 and 87 years (median, 61 years; IQR, 48–70 years). The seropositivity proportion was largest for people aged 65 years or more (30 of 192; unweighted proportion, 15.6%; weighted proportion, 13.7%) (Box 3). No participants under 20 years of age were seropositive (Box 4).
The unweighted seropositivity proportions were similar for male (30 of 358, 8.4%) and female participants (50 of 559, 9.0%), but the weighted proportion was larger for male participants (10.6% v 7.5%). For Aboriginal or Torres Strait Islander participants, the unweighted (5 of 42, 12%) and weighted seropositivity proportions (26%) were larger than for all participants, but the absolute number of positive tests was small. Weighted estimates by town ranged between 2.0% (Temora) and 11.6% (Griffith) (Box 3).
The only statistically significant associations between seropositivity and possible risk factors and protective measures were reduced prevalence for people who reported picnicking (POR, 0.5; 95% CI, 0.2–0.9) or camping as recreational activity (POR, 0.5; 95% CI, 0.2–0.9), and increased prevalence with no water body near residence (POR, 2.1; 95% CI, 1.1–3.7) (Supporting Information, table).
Discussion
We found evidence for a substantial number of JEV infections in five regional NSW towns during a single arbovirus season in 2022, based on detection of JEV‐specific antibody (single high titres). Prior to 2022 in NSW, Murray Valley encephalitis and Kunjin viruses were monitored in a program of sentinel chicken and mosquito surveillance, but not JEV. In Australia, active JEV surveillance has only been undertaken (intermittently) in the Torres Strait Islands.17 Our findings provide information relevant to local prevention strategies, including vaccination and mosquito avoidance advice.
We surveyed communities deemed to be at risk of JEV infection, based on ecological risk factors and notified cases of clinical Japanese encephalitis. Greater rainfall across the eastern seaboard of Australia during the La Niña period resulted in favourable conditions for large increases in the numbers of both vector (mosquitoes) and host (waterbirds) immediately before our study.18 The overall proportion of seropositive samples was 8.7%, but the proportion differed by township. Ecological factors and our seropositivity findings suggest that large proportions of these communities were at risk of JEV infection, but additional public health control measures since the 2022 outbreak probably minimised the number of infections causing clinical disease.
In a JEV‐naïve population, the risk of infection would be similar for children and adults.19 We did not recruit sufficient numbers of children to compare the proportion of seropositive results with those for adults, but the unweighted and weighted proportions of seropositive results for adults increased with age. This might be explained by older people spending more time outdoors; it is unlikely to reflect serologic cross‐reactivity with antibody to other flaviviruses, given the high specificity of our assay. National surveillance data indicated that four of 41 cases of Japanese encephalitis in Australia during 2022 were in children, with a lower incidence than in older age groups.20 The median age of people with notified Japanese encephalitis during January 2021 – June 2022 was 61.5 years, and most were male.8 In our serosurvey, the weighted proportion of seropositive results was slightly greater for male than female participants.
We found that the estimated prevalence of JEV infection was about twice as high for people whose homes were not close to any body of water as for those who lived closer to water bodies, but the public health significance of this finding is unclear. We also found that the estimated prevalence was lower for people who went camping or on picnics, and were therefore potentially exposed to JEV‐carrying mosquitoes; these findings may reflect misclassification bias, confounding, or people taking protective precautions during outdoor activities. As we did not adjust our analyses for recreation undertaken outside high‐risk areas, participants may have undertaken such activities elsewhere during the study period. The sample size in our study may have been inadequate for assessing the influence of some exposure factors because many are common for people in rural and regional areas. Exposure risk may also differ between towns because of differing proximity to large commercial piggeries, for example, and other, unmeasured factors.
Our detection of evidence of JEV infections in southeastern Australia indicates a significant expansion of the known risk areas for Japanese encephalitis, possibly reflecting the impact of climate change and an associated increase in the risk of mosquito‐borne diseases. The specific ecologic and geographic risk factors that underlie this spread are still unclear, but are probably complex and specific to the ecologically diverse and vast land mass of Australia.21 The complex interplay of vector, host, and environmental factors in JEV transmission requires a One Health disease management strategy.22 Mosquito and animal surveillance has been substantially intensified since the 2022 outbreak, together with multidisciplinary collaboration across health sectors.23 Climate change and climate change adaptation each require integrated approaches to managing the risks of vector‐borne disease and outbreak control.24
Until now, JEV vaccination was primarily available in Australia only to people travelling to countries in which the virus is endemic. As vaccine stocks were quite limited, a phased approach based on highest risk of exposure was required.25,26 The National JEV Vaccination Plan, adopted in August 2022, includes additional eligibility criteria related to local risk information, vaccine availability, and other state‐ or territory‐specific factors.25,26 Our serosurvey findings were considered by state and national expert panels when developing this plan. In NSW, mosquito avoidance strategies remain central to controlling JEV outbreaks, given limited vaccination coverage and other circulating non‐vaccine‐preventable arboviruses.27
Limitations
We collected more detailed demographic, history, and risk information about our serosurvey participants than is available to serosurveys based on residual sera from blood donors. Weighting our prevalence estimates mitigated some limitations associated with convenience sampling. Nevertheless, our findings may not be generalisable to all people in the five towns we examined, nor to all people in NSW. Our study design entailed risks of misclassification bias and confounding. The number of study sites was limited by our resources, reducing the statistical power of our analyses. However, our rapid results were important for immediate public health response planning and contributed to broader national analyses and virus transmission risk modelling.
Conclusion
We report the first community‐based serosurvey in southeastern Australia following an outbreak of Japanese encephalitis. It provided timely and important information for vaccination policy and public health actions. About one in eleven survey participants in five NSW towns at high risk were infected with JEV during a single arbovirus season. Public health responses, including effective surveillance, vaccination against JEV, and mosquito management, are critical for controlling outbreaks. Promoting behaviours that reduce exposure to mosquitoes is a core component of prevention, particularly during outbreaks, when the vaccine supply may be limited.
Box 1 – New South Wales local government areas in which cases of Japanese encephalitis were reported during the 2022 outbreak, and where our serosurvey was undertaken, 21 June – 22 July 2022
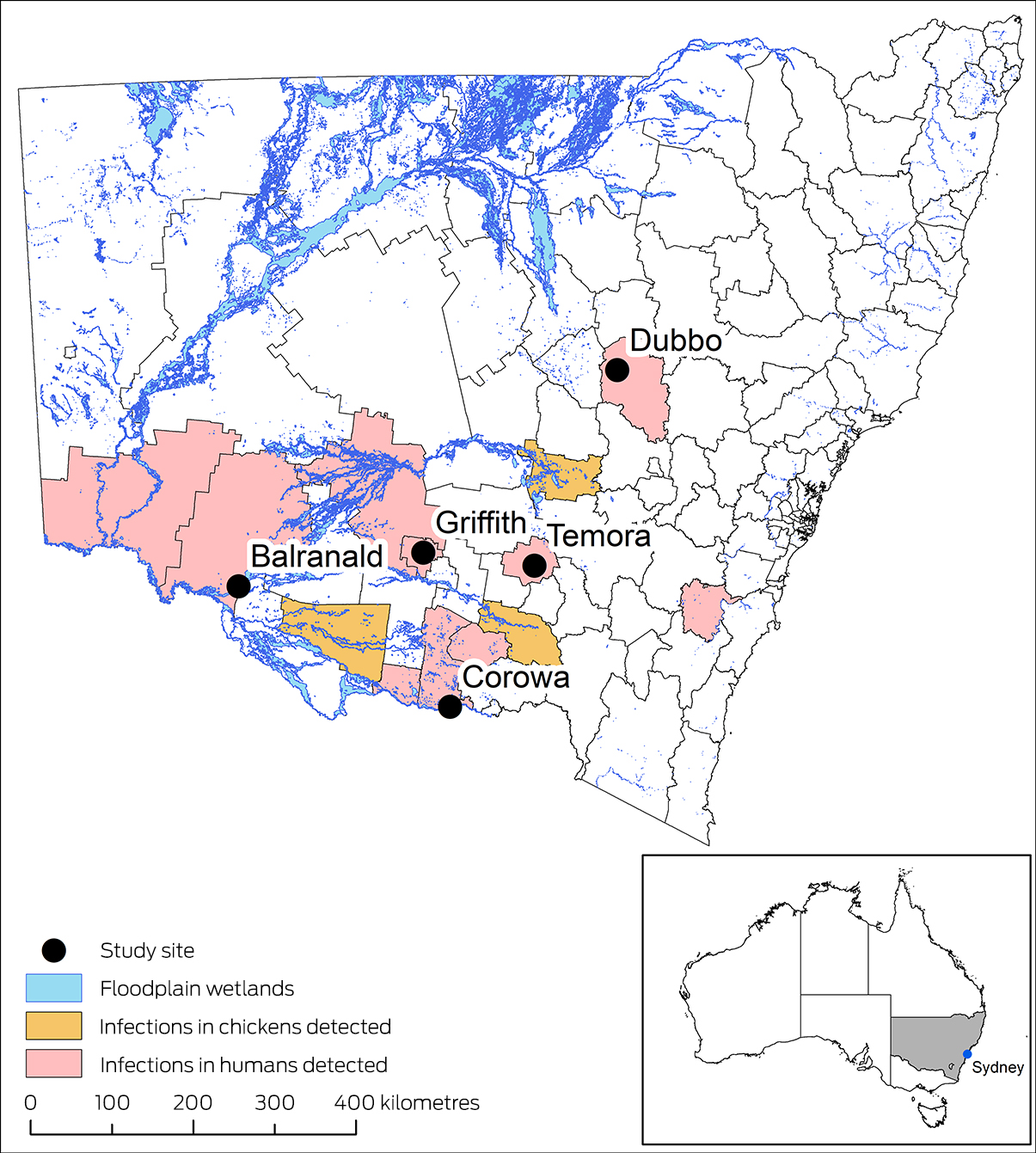
Data sources: NSW Department of Climate Change, Energy, the Environment and Water (SEED database);13 Australian Bureau of Statistics boundary files.14 Coordinate Reference System (CRS): GDA2020 NSW Lambert.
Box 2 – Characteristics of the Japanese encephalitis virus‐specific total antibody prevalence serosurvey participants and of their source populations
|
Characteristic |
Survey participants |
Source population* |
|||||||||||||
|
|
|||||||||||||||
|
All people |
917 |
88 806 |
|||||||||||||
|
Age (years) |
|
|
|||||||||||||
|
0–19 |
27 (2.9%) |
23 223 (26.1%) |
|||||||||||||
|
20–34 |
178 (19.4%) |
17 452 (19.6%) |
|||||||||||||
|
35–54 |
304 (33.2%) |
21 107 (23.7%) |
|||||||||||||
|
55–64 |
216 (23.6%) |
10 810 (12.1%) |
|||||||||||||
|
65 or older |
192 (20.9%) |
16 385 (18.4%) |
|||||||||||||
|
Sex |
|
|
|||||||||||||
|
Male |
358 (39.0%) |
43 699 (49.2%) |
|||||||||||||
|
Female |
559 (61.0%) |
45 114 (50.8%) |
|||||||||||||
|
Aboriginal or Torres Strait Islander people |
42 (4.6%) |
8783 (9.9%) |
|||||||||||||
|
Town |
|
|
|||||||||||||
|
Balranald |
60 (6.5%) |
1452 (1.6%) |
|||||||||||||
|
Corowa |
142 (15.5%) |
7050 (7.9%) |
|||||||||||||
|
Dubbo |
260 (28.4%) |
46 078 (51.9%) |
|||||||||||||
|
Griffith |
308 (33.6%) |
28 126 (31.7%) |
|||||||||||||
|
Temora |
147 (16.0%) |
6100 (6.9%) |
|||||||||||||
|
|
|||||||||||||||
|
* Source: Australian Bureau of Statistics.15 The Australian Bureau of Statistics makes small random adjustments to all reported cell values in their mesh block data to protect confidentiality.16 These adjustments may cause the sum of rows or columns to differ by small amounts from table totals. |
|||||||||||||||
Box 3 – Japanese encephalitis virus‐specific total antibody seropositivity, unweighted and weighted, by age group, sex, and Indigenous status
|
|
Seropositive results |
||||||||||||||
|
Characteristic |
Number |
Unweighted proportion |
Weighted proportion |
||||||||||||
|
|
|||||||||||||||
|
Total |
80/917 |
8.7% |
— |
||||||||||||
|
Age (years) |
|
|
|
||||||||||||
|
0–19 |
0/27 |
— |
— |
||||||||||||
|
20–34 |
12/178 |
6.7% |
8% |
||||||||||||
|
35–54 |
19/304 |
6.3% |
4.5% |
||||||||||||
|
55–64 |
19/216 |
8.8% |
4.5% |
||||||||||||
|
65 or older |
30/192 |
15.6% |
13.7% |
||||||||||||
|
Sex |
|
|
|
||||||||||||
|
Male |
30/358 |
8.4% |
10.6% |
||||||||||||
|
Female |
50/559 |
9.0% |
7.5% |
||||||||||||
|
Aboriginal or Torres Strait Islander people |
5/42 |
12% |
26% |
||||||||||||
|
Town |
|
|
|
||||||||||||
|
Balranald |
16/60 |
27% |
6.7% |
||||||||||||
|
Corowa |
10/142 |
7.0% |
3.6% |
||||||||||||
|
Dubbo |
9/260 |
3.5% |
6.3% |
||||||||||||
|
Griffith |
38/308 |
12.3% |
11.6% |
||||||||||||
|
Temora |
7/147 |
4.8% |
2.0% |
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 25 August 2023, accepted 27 November 2023
- Zoe Baldwin1,2
- Linda Hueston3
- April Roberts‐Witteveen4
- Priscilla Stanley5
- Meru Sheel6
- Noni Winkler7
- Archana Koirala7,8
- Kristine Macartney7,9
- Jennifer Case10
- Kirsty Hope10
- Keira M Glasgow10
- 1 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT
- 2 Hunter New England Local Health District, Newcastle, NSW
- 3 CIDMLS Institute of Clinical Pathology and Medical Research, Sydney, NSW
- 4 Southern and Murrumbidgee Local Health District, Goulburn, NSW
- 5 Western New South Wales Local Health District, Dubbo, NSW
- 6 Sydney School of Public Health, University of Sydney, Sydney, NSW
- 7 National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, Sydney, NSW
- 8 Nepean Hospital, Nepean Blue Mountains Local Health District, Penrith, NSW
- 9 The University of Sydney, Sydney, NSW
- 10 New South Wales Department of Health, Sydney, NSW
Open access:
Open access publishing facilitated by Australian National University, as part of the Wiley ‐ Australian National University agreement via the Council of Australian University Librarians.
Data sharing:
Individual participant data for this study are not available for sharing.
This study was funded by NSW Health. Zoe Baldwin is a MPhil (Applied Epidemiology) Scholar with the National Centre for Epidemiology and Population Health at the Australian National University, supported by an MPhil (Applied Epidemiology) Scholar research stipend from NSW Health.
This research was conducted in collaboration with the National Centre for Immunisation Research and Surveillance, NSW Health Pathology, and the Institute of Clinical Pathology and Medical Research at Westmead Hospital. We acknowledge the operational support provided by Sarah Davies, Chloe Luscombe, and Adriana Notaras (NSW Health), and others in the Southern NSW, Murrumbidgee, Western NSW, and Far Western NSW public health units.
Open access publishing facilitated by Australian National University, as part of the Wiley ‐ Australian National University agreement via the Council of Australian University Librarians.
No relevant disclosures.
- 1. World Health Organization. Japanese encephalitis. 9 May 2019. https://www.who.int/news‐room/fact‐sheets/detail/japanese‐encephalitis (viewed Nov 2022).
- 2. Hills SL, Netravathi M, Solomon T. Japanese encephalitis among adults: a review. Am J Trop Med Hyg 2023; 108: 860‐864.
- 3. Australian Department of Health and Aged Care. People who live or work on the outer islands of Torres Strait are recommended to receive JE vaccine. Updated 5 June 2018. https://immunisationhandbook.health.gov.au/recommendations/people‐who‐live‐or‐work‐on‐the‐outer‐islands‐of‐torres‐strait‐are‐recommended‐to‐receive‐je‐vaccine (viewed Nov 2022).
- 4. Australian Department of Health and Aged Care. Japanese encephalitis. Updated 19 May 2022. https://immunisationhandbook.health.gov.au/contents/vaccine‐preventable‐diseases/japanese‐encephalitis (viewed Nov 2022).
- 5. Waller C, Tiemensma M, Currie BJ, et al. Japanese encephalitis in Australia: a sentinel case. N Engl J Med 2022; 387: 661‐662.
- 6. Australian Department of Agriculture, Fisheries and Forestry. Japanese encephalitis. Updated 4 Dec 2023. https://www.agriculture.gov.au/biosecurity‐trade/pests‐diseases‐weeds/animal/japanese‐encephalitis (viewed May 2023).
- 7. New South Wales Health. Japanese encephalitis: update. 24 Mar 2022. https://www.health.nsw.gov.au/environment/pests/vector/Documents/je‐virus‐gp‐update‐24032022.pdf (viewed Nov 2023).
- 8. Reyes A, Kane S, Glynn‐Robinson A. Emergence of locally acquired JEV in Australia from January 2021 – June 2022. Proceedings of the Communicable Diseases and Immunisation Conference, 19 June 2023, Perth, Western Australia [unpublished abstract/presentation].
- 9. Gillett Z, Taschetto A. Multi‐year La Niña events [ARC Centre of Excellence for Climate Extremes briefing note 20]. Australian Research Council, 2022. https://climateextremes.org.au/wp‐content/uploads/Multi‐year‐La‐Nina‐Events‐ARC‐Centre‐of‐Excellence‐for‐Climate‐Extremes.pdf (viewed July 2023).
- 10. Hawkes RA, Boughton CR, Naim HM, et al. Arbovirus infections of humans in New South Wales: seroepidemiology of the flavivirus group of togaviruses. Med J Aust 1985; 143: 555‐561.
- 11. Winkler NE, Koirala A, Kaur G, et al; Australian Japanese Encephalitis Virus Serosurvey Group. Seroprevalence of Japanese encephalitis virus‐specific antibodies in Australia following novel epidemic spread: protocol for a national cross‐sectional study. BMJ Open 2024; 14: e075569.
- 12. Von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344‐349.
- 13. NSW Department of Climate Change, Energy, the Environment and Water. NSW Wetlands. Updated 10 Jan 2010. https://datasets.seed.nsw.gov.au/dataset/36c734bd‐1c9c‐40b9‐966a‐0ad0f7500a09 (viewed Nov 2023).
- 14. Australian Bureau of Statistics. Digital boundary files. Australian Statistical Geography Standard (ASGS) edition 3; July 2021 – June 2026. 20 July 2021. https://www.abs.gov.au/statistics/standards/australian‐statistical‐geography‐standard‐asgs‐edition‐3/jul2021‐jun2026/access‐and‐downloads/digital‐boundary‐files (viewed Nov 2023).
- 15. Australian Bureau of Statistics. GeoPackages. Census data tools and products. 12 Apr 2022. https://www.abs.gov.au/census/guide‐census‐data/about‐census‐tools/geopackages (viewed Oct 2022).
- 16. Australian Bureau of Statistics. Confidentiality and relative standard error, TableBuilder. 19 Nov 2021. https://www.abs.gov.au/statistics/microdata‐tablebuilder/tablebuilder/confidentiality‐and‐relative‐standard‐error (viewed Nov 2023).
- 17. Australian Department of Agriculture, Fisheries and Forestry. Significant events in the history of NAQs. Historical events before the establishment of the Northern Australia Quarantine Strategy. Updated 26 Nov 2020. https://www.agriculture.gov.au/biosecurity‐trade/policy/australia/naqs/significant‐events (viewed July 2023).
- 18. Walsh MG, Webb C, Brookes V. An evaluation of the landscape structure and La Niña climatic anomalies associated with Japanese encephalitis virus outbreaks reported in Australian piggeries in 2022. One Health 2023; 16: 100566.
- 19. Campbell GL, Hills SL, Fischer M, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89:766‐774, 774A‐774E.
- 20. Australian Department of Health and Aged Care. National Notifiable Disease Surveillance System surveillance dashboard. https://nindss.health.gov.au/pbi‐dashboard (viewed May 2023).
- 21. Connor B, Bunn WB. The changing epidemiology of Japanese encephalitis and new data: the implications for new recommendations for Japanese encephalitis vaccine. Trop Dis Travel Med Vaccines 2017; 3: 14.
- 22. Impoinvil DE, Baylis M, Solomon T. Japanese encephalitis: on the One Health agenda. Curr Top Microbiol Immunol. 2013; 365: 205‐247.
- 23. Department of Health and Aged Care. $69 million for Japanese encephalitis virus (JEV) response [ministerial media release]. 11 Mar 2022. https://www.health.gov.au/ministers/the‐hon‐greg‐hunt‐mp/media/69‐million‐for‐japanese‐encephalitis‐virus‐jev‐response (viewed June 2022).
- 24. Zinsstag J, Crump L, Schelling E, et al. Climate change and One Health. FEMS Microbiol Lett 2018; 365: fny085.
- 25. New South Wales Health Japanese encephalitis: information for health professionals. Updated 28 Nov 2023. https://www.health.nsw.gov.au/Infectious/jev/Pages/health‐professionals.aspx#vax‐approach (viewed Nov 2023).
- 26. Biocelect. IMOJEV: Japanese encephalitis vaccine, live attenuated [media release]. 20 Feb 2023. https://www.biocelect.com/post/imojev‐japanese‐encephalitis‐vaccine‐live‐attenuated (viewed Nov 2023).
- 27. New South Wales Health. NSW expands access to Japanese encephalitis vaccine [press release]. 14 Sept 2022. https://www.health.nsw.gov.au/news/Pages/20220914_01.aspx (viewed Oct 2022).






Abstract
Objectives: To determine the proportion of people in New South Wales towns at high risk of Japanese encephalitis virus (JEV) infections during the 2022 outbreak; to identify risk factors for JEV infection.
Study design: Cross‐sectional serosurvey study of the seroprevalence of JEV‐specific antibodies in NSW.
Setting, participants: Convenience sample of people (all ages) from five regional NSW towns deemed to be at high risk of JEV infections after first outbreak of Japanese encephalitis in southeastern Australia in early 2022 (Balranald, Corowa, Dubbo, Griffith, Temora), 21 June – 22 July 2022.
Main outcome measures: Proportion of people seropositive for JEV total antibody, assayed by defined epitope‐blocking enzyme‐linked immunosorbent assay; prevalence odds ratios for exposure risk factors and protective behaviours.
Results: Eighty of 917 eligible participants (559 girls or women, 61%; 42 Aboriginal and Torres Strait Islander people, 4.6%; median age, 52 years [IQR, 37–62 years]) were seropositive for JEV‐specific total antibody (8.7%); the median age of seropositive people was 61 years (IQR, 48–70 years). The seropositivity proportion was largest for people aged 65 years or more (30 of 192; weighted proportion, 13.7%) and larger for male than female participants (30 of 358, 10.6%v 50 of 559, 7.5%). Five of 42 samples from Aboriginal and Torres Strait Islander participants were seropositive (12%). We found mixed associations with a range of potential risk factors.
Conclusion: We found evidence for a substantial number of JEV infections in five regional NSW towns during a single arbovirus season in 2022. Public health responses, including effective surveillance, vaccination against JEV, and mosquito management, are critical for controlling outbreaks. Promoting behaviours that reduce exposure to mosquitoes is a core component of prevention, particularly when the vaccine supply is limited.