Clinical record
A 61‐year‐old man with moderate stroke risk factors, including hypertension and hyperlipidaemia, presented with rapidly progressive ptosis, diplopia, ataxia and dysarthria. No infective prodrome or suspected food poisoning was initially disclosed. Given this presentation, brainstem stroke was the primary differential diagnosis.
On Day 2, he developed vomiting, dysphagia and severe respiratory distress, requiring intubation. He subsequently developed complete ophthalmoplegia, descending flaccid paralysis and required ventilation. A differential diagnosis of Miller Fisher syndrome was then considered. Sequential intravenous immunoglobulin and plasma exchanges were minimally effective. Cerebrospinal fluid analysis was unremarkable with no raised protein levels. Test results for anti‐ganglioside antibodies, including anti‐GQ1‐b (both IgG and IgM), were negative. Nerve conduction studies and electromyogram (performed on Day 4) results confirmed a generalised, predominantly motor neuropathy (Box 1 and Box 2). Results from the magnetic resonance imaging (MRI) scan of the brain and spine/plexus with contrast were normal.
Further history on Day 15 revealed that the patient had consumed foul‐tasting almond milk 12–36 hours prior to symptom onset. The differentials then expanded to include botulism. Following consultation with the infectious diseases department, further tests were requested. Clostridium botulinum culture and test results for toxin A‐G nucleic acid were negative on retained milk sample and stool. The results from the C. botulinum direct toxin test using the mouse bioassay (pooled antitoxin A, B and E) demonstrated the presence of botulinum toxin in a retained sample of the milk. The FilmArray BioThreat Panel (BioFire Defense) test on a retained sample of milk detected botulinum toxin A nucleic acid. After guidance from infectious diseases and public health authorities on Day 16, the patient was administered botulin antitoxin obtained from the National Medical Stockpile. The implicated milk product was subsequently recalled.1 The patient was weaned off mechanical ventilation five months after admission to the intensive care unit.
Discussion
Botulism is a rare, life‐threatening disease caused by neurotoxins produced by C. botulinum (and rarely, by Clostridium baratii and Clostridium butyricum). C. botulinum is a ubiquitous gram‐positive, spore‐forming obligate anaerobic bacterium.2 Its spores germinate under conditions such as in improperly processed, canned, low acid or alkaline foods where anaerobic conditions have occurred. Botulin toxins are extremely potent; they induce paralysis by binding to presynaptic acetylcholine receptors and preventing neurotransmitter release. Toxins A, B, E and F cause disease in humans, with toxin A associated with the most severe toxidrome. Common routes of exposure include ingestion (foodborne botulism), colonisation of a wound (wound botulism), the intestines (infant botulism), and via high concentration cosmetic or therapeutic injections of toxin (iatrogenic botulism). Inhalational botulism has also occurred among laboratory workers.2
Botulin antitoxin is the only specific therapy available for botulism. If diagnosis is suspected, antitoxin should be administered urgently as it can prevent the progression of paralysis, although it cannot reverse existing paralysis.2 Antitoxin is most effective if given within 48 hours of symptom onset; in adult foodborne botulism, the decision to administer antitoxin after 72 hours is a clinical one, with limited evidence of an upper limit on the timing of antitoxin administration after which it would offer no benefit.2,3
Foodborne botulism is very rare in Australia; this was the first case linked to a commercially prepared product since 2007. Following notification of this case of suspected foodborne botulism, the manufacturer of the product issued a national recall due to missing storage instructions: the product had not been labelled with directions to “keep refrigerated”.4 The classic presentation is acute onset of bilateral cranial neuropathies with associated symmetrical descending weakness.2 These symptoms include blurred vision, diplopia, nystagmus, ptosis, dysphagia, dysarthria and facial weakness.2 Descending muscle weakness usually progresses from the trunk and upper extremities to lower extremities.2 Smooth muscle failure in this setting can also cause urinary retention and constipation.2 Patients are frequently afebrile with normal mental status and sensation.2 In foodborne botulism, prominent gastrointestinal symptoms such as nausea and vomiting often accompany onset of weakness.
Respiratory failure due to botulism often requires intubation and mechanical ventilation, as in the case with our patient.2 This is due to diaphragmatic paralysis from descending muscle weakness. Patients whose condition deteriorates to respiratory failure frequently require prolonged hospitalisation for weeks to months, although patients with less severe illness may have a faster recovery.5 Long term morbidity is low in patients with mild disease, with complete resolution of symptoms within three months.5 However, patients with severe disease may experience years of neurological deficits and sequelae from extended mechanical ventilation.5
This case was diagnostically challenging due to an initially limited history with rapidly progressive signs that overlapped between botulism, Miller Fisher syndrome and brainstem stroke. Furthermore, there may be considerable delay in diagnosis as some investigations that can distinguish between these differentials have a prolonged turnaround time and poor diagnostic accuracy. If botulism is suspected, antitoxin should be administered urgently.
Lessons from practice
- Clinical features of botulism, Miller Fisher syndrome and brainstem strokes frequently overlap and are often rapidly progressive. Differentiation may be improved by recognising the absence of sensory features and the pattern of evolving weakness (ascending in Guillain–Barré syndrome, descending with early cranial nerve involvement with Miller Fisher syndrome and botulism), as well as the tempo of symptom onset and evolution (hyperacute with strokes versus acute and often progressive with Miller Fisher syndrome and botulism).
- Investigations to distinguish between these conditions are not always rapidly available or reliable.
- If botulism is suspected, timely administration of antitoxin is critical. Botulism antitoxin is available from the National Medical Stockpile.
Box 1 – Day 4 results from the nerve conduction study and electromyogram suggesting a severe motor predominant neuropathy with relative sensory sparing (likely too early to appreciate muscle denervation)
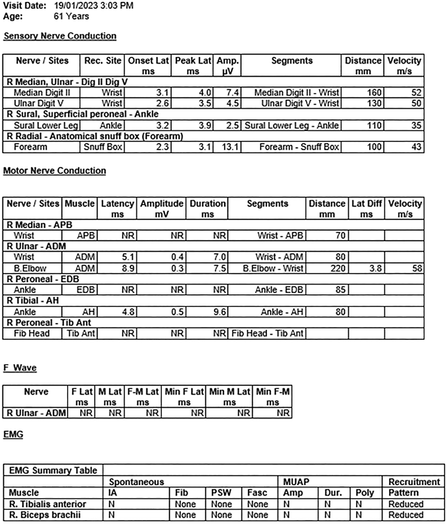
ADM = abductor digiti minimi; AH = abductor halluces; Amp. = amplitude; APB = abductor pollicus brevis; Diff = difference; Dig. = digit; Dur. = duration; EDB = extensor digitorum brevis; EMG = electromyogram; F = F wave; F‐M = F wave minimum; Fasc = fasciculations; IA = insertional activity; Lat. = latency; Min = minimum; MUAP = motor unit action potential; N = normal; NR = not recordable; Poly = polyphasia; PSW = positive sharp waves; R = recorded site; Tib Ant = tibialis anterior muscle.
Box 2 – Day 88 results from the nerve conduction study and electromyogram showing persisting generalised reduction in upper and lower limb motor amplitudes, (although improving), with relative sparing of sensory responses
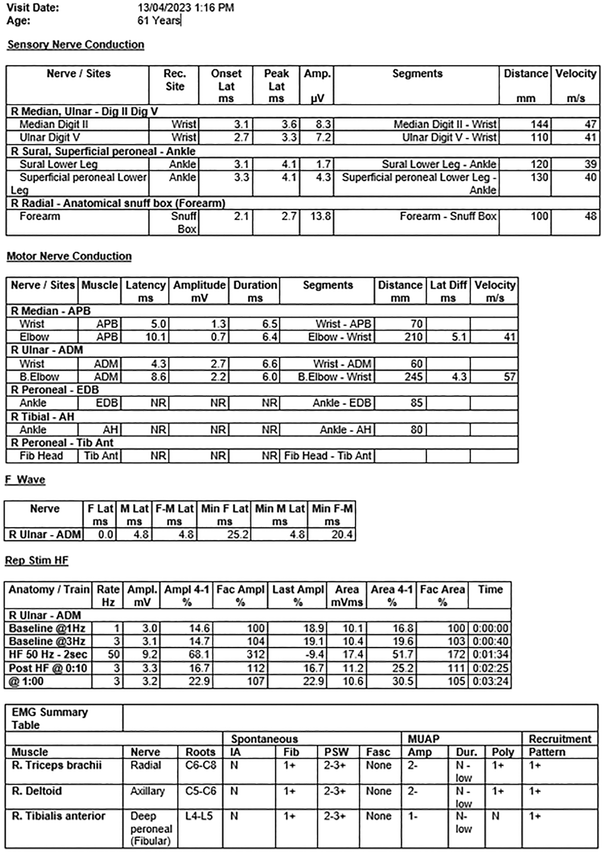
ADM = abductor digiti minimi; AH = abductor halluces; Amp. = amplitude; APB = abductor pollicus brevis; Diff = difference; Dig. = digit; EDB = extensor digitorum brevis; EMG = electromyogram; F = F wave; F‐M = F wave minimum; Fasc = fasciculations; HF = high frequency stimulation; IA = insertional activity; Lat. = latency; Min = minimum; MUAP = motor unit action potential; N = normal; NR = not recordable; Poly = polyphasia; Rec. = recorded site; Rep Stim HF = high frequency repetitive stimulation study; Tib Ant = tibialis anterior muscle.
Provenance: Not commissioned; externally peer reviewed.
- 1. NSW Health. Recall of long life almond milk product [media release]. 16 Feb 2023. https://www.health.nsw.gov.au/news/Pages/20230216_00.aspx#:~:text=NSW%20Health%20is%20advising%20people,sale%20at%20Woolworths%20in%20NSW (viewed Dec 2023).
- 2. Rao AK, Sobel J, Chatham‐Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021; 70: 1‐30.
- 3. NSW Health. Botulism control guideline. 2015. https://www.health.nsw.gov.au/Infectious/controlguideline/Pages/botulism.aspx (viewed May 2023).
- 4. NSW Food Authority. Recall UPDATE: JS Health x Inside Out Almond Milk and Oat Milk 1 L [media release]. 16 Feb 2023. https://www.foodauthority.nsw.gov.au/news/recalls/update‐js‐health‐x‐inside‐out‐almond‐milk‐and‐oat‐milk‐1‐l (viewed Dec 2023).
- 5. Souayah N, Mehyar LS, Khan HM, et al. Trends in outcome and hospitalization charges of adult patients admitted with botulism in the United States. Neuroepidemiology 2012; 38: 233‐236.





Patient consent:
The patient provided written consent for publication.
No relevant disclosures.