The known: Infections are serious complications of cardiac implantable electronic device (CIED) procedures that cause significant morbidity and mortality.
The new: Of 37 675 people who underwent CIED procedures in NSW during 2016–21, 397 (1.1%) were hospitalised with related infections within twelve months, and 500 (1.3%) by the end of follow‐up (median, 27 months). For people receiving CIED replacements or upgrades or new cardiac resynchronisation therapy with defibrillators, the respective proportions were 1.5% and 2.5%. A range of patient, device, and procedural factors were associated with greater risk of CIED‐related infection.
The implications: Awareness of specific risk factors for CIED‐related infections is needed for decisions about CIED procedures and prophylactic measures.
Cardiac implantable electronic device (CIED)‐related infections are serious procedure‐related complications that increase the risk of death,1,2 and their management often requires hospitalisation, and usually system removal.3,4 To prevent these infections, the American Heart Association recommends (class I) prophylactic intravenous antibiotic treatment (typically cefazolin) and meticulous attention to sterilisation.5 The number of CIED‐related infections is nevertheless rising as the number of people receiving CIEDs increases.6 A range of infection rates have been reported.2,6,7 Recent multicentre clinical trials8,9 have found overall 12‐month infection rates of about 1%, while large population‐based studies have reported rates between 0.7% and 4.3%.7 The device type, procedure type, and follow‐up time each influence the CIED‐related infection rate,6 and all need to be considered when comparing infection rates.
As CIED‐related infections are associated with poorer patient outcomes,1,2 identifying patient, device, and procedural factors that increase the risk of infection is crucial for developing clinical strategies that minimise the risk. Current understanding of risk factors is based primarily on studies in single centres or with interventional designs;8,9,10 only limited relevant population‐level data are available. Moreover, many studies have examined only new implant procedures, but the numbers of revision and replacement procedures are increasing.6,7
Information about CIED‐related infections in Australia is limited.11,12,13 The most recent analysis was of data for 2010–2015,11 and there have been no reports on factors associated with CIED‐related infections. We therefore quantified the rate of CIED‐related infections and investigated risk factors for such infections by examining hospital admissions data for New South Wales for the period 2016–21.
Methods
For our retrospective cohort study, we analysed person‐level linked hospital (Admitted Patient Data Collection, APDC) and mortality data for NSW residents, 1 January 2016 to 30 June 2021 (public hospitals) or 30 June 2020 (private hospitals; private hospital APDC data collection is often slower than for public hospitals). APDC records include demographic characteristics, diagnoses, and procedures for all public and private hospital admissions in NSW. Diagnoses are coded according to the International Statistical Classification of Diseases, tenth revision, Australian modification (ICD‐10‐AM), procedures according to the Australian Classification of Health Interventions (ACHI).14
We identified CIED procedures for adults (18 years or older) by ACHI codes in the primary and all secondary procedure fields in the APDC (Supporting Information, table 1). When several related procedures (eg, insertion of a generator followed by insertion of a lead) were undertaken during a hospital stay, only one was counted to avoid double counting. If multiple hospital stays for CIED procedures during the study period were identified for an individual, only the first CIED procedure was included. Admissions were excluded if admission and death dates were inconsistent, or only CIED removal or pocket procedures were undertaken.
CIED devices were classified as permanent pacemakers (PPMs), implantable cardioverter–defibrillators (ICDs), cardiac resynchronisation therapy with pacemaker (CRT‐P), or cardiac resynchronisation therapy with defibrillator (CRT‐D). CRT devices were identified by procedures involving left ventricular lead implantation. CIED procedures were classified as new, revision, or replacement (including upgrade) procedures.
The rate of CIED‐related infections was also estimated for people at high risk of such infections, as defined by the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP‐IT):9 people who underwent CIED replacement or system upgrade with or without new leads, or a new CRT‐D procedure.
Study outcome
The primary outcome was hospitalisation with a CIED‐related infection after the CIED procedure. An ICD‐10‐AM code specific for CIED‐related infection (T82.71), introduced on 1 July 2017, in any diagnosis field identified infections from this date onwards. CIED infections prior to 1 July 2017 were identified by a more general code for cardiac device‐related infections (T82.7) and either a CIED removal procedure or the status code for the presence or management of a CIED (Z95.0; Z45.0) in the procedure or diagnosis fields for an episode of care. This definition yielded annual numbers of infections prior to July 2017 broadly consistent with those for infections from July 2017.
Statistical analysis
We followed patients from their CIED procedure date until the earliest of three endpoints: first hospital admission with a CIED‐related infection, death, or end of follow‐up (30 June 2020 for private hospitals, 30 June 2021 for public hospitals). We depict the cumulative incidence of CIED‐related infections in Kaplan–Meier plots. We report incidence rates per 1000 person‐months by device type and procedure type, and for specific follow‐up periods after the CIED procedure. To maintain patient anonymity, we do not report infection numbers lower than five in tables.
We summarise data for continuous variables as means with standard deviations (SDs), for categorical variables as counts and proportions. The statistical significance of differences in the characteristics of people with or without CIED‐related infections were assessed in 2‐sample Student t or Mann–Whitney tests (continuous variables) or χ2 tests (categorical variables).
We investigated associations with CIED‐related infections of the following factors — selected according to data from the PADIT (Prevention of Arrhythmia Device Infection Trial)15 and in consultation with clinicians — in Cox proportional hazards regression models: age, sex, private insurance status, emergency admission, CIED device type, CIED procedure type, number of prior CIED procedures, previous CIED infection, other medical conditions, concomitant cardiac surgery, prior coronary artery bypass or valve surgery, and hospital type. Medical conditions — diabetes, hypertension, coronary artery disease, congestive heart failure, atrial fibrillation, cardiomyopathy, peripheral vascular disease, stroke, chronic obstructive pulmonary disease (COPD), sleep apnoea, chronic kidney disease, and obesity — were identified by ICD‐10‐AM codes in diagnosis fields for the CIED procedure hospitalisation or another hospitalisation in the twelve months preceding the CIED procedure (Supporting Information, tables 2 and 3). The number of previous CIED procedures was determined from hospital records for admissions between 1 July 2001 and the admission included in our analysis. We initially evaluated associations between variables and CIED‐related infection in unadjusted analyses and minimally adjusted analyses (adjusted for age, sex, other medical condition). To determine which factors were most strongly associated with CIED‐related infections (noting correlations between variables such as number of prior procedures, procedure type, and prior CIED infection), we included all covariates for which P < 0.10 in the unadjusted models and then successively removed covariates for which P > 0.05 using the Hosmer–Lemeshow stepwise backward elimination approach.16 We report adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). The proportional hazards assumption was assessed by checking the scaled Schoenfeld residuals; collinearity was assessed with the variance inflation factor. All analyses were conducted in Stata 16.0.
Ethics approval
This study was approved by the human research ethics committees of the University of New South Wales (RG171323), NSW Population and Health Services Research (HREC/18/CIPHS/56), the Aboriginal Health and Medical Research Council of NSW (1503/19), and the Australian Institute of Health and Welfare (EO2018/2/431).
Results
Of 60 428 CIED procedures in NSW during 2016–21 (public hospitals) or 2016–20 (private hospitals), 37 675 were included in our analysis (23 194 men, 63.5%) (Supporting Information, figure 1). A total of 500 CIED‐related infections were identified; the median follow‐up time was 24.9 months (interquartile range, 11.2–40.8 months) (Supporting Information, figure 2). Compared with people who did not have CIED‐related infections, the mean age of those who did was slightly higher (75.8 [SD, 12.8] v. 72.2 [SD, 15.2] years), and larger proportions had devices with CRT capabilities (15.2% v. 8.2%), had undergone replacement or revision procedures (34.4% v 26.5%) or concomitant cardiac procedures (5.6% v 3.4%), had previously undergone valve surgery (4.6% v 2.4%), or had a variety of other medical conditions. The proportions of people who had previously undergone CIED procedures (30.0% v 18.7%) or had CIED‐related infections (12.2% v 0.8%) were also larger for people who had infections following the index procedure (Box 1).
Incidence of CIED‐related infection
Within twelve months of their index CIED procedures, 397 people had experienced CIED‐related infections (Kaplan–Meier rate, 1.1%), and 500 by the end of follow‐up (Kaplan–Meier rate, 1.3%) (Box 2); 148 of 10 540 people at high risk of CIED‐related infections had experienced CIED‐related infections within twelve months (Kaplan–Meier rate, 1.5%) and 186 by the end of follow‐up (Kaplan–Meier rate, 2.5%) (Supporting Information, figure 3). By device type, the incidence rate was highest following CRT‐D device procedures (45 of 1482 people, Kaplan–Meier rate, 3.4%) and lowest after PPM procedures (302 of 27 416, Kaplan–Meier rate, 1.3%); by procedure type, it was highest following revision procedures (157 of 9419, Kaplan–Meier rate, 3.2%) and lowest following new insertions (328 of 27 325; Kaplan–Meier rate, 1.2%) (Box 2).
The overall infection rate was 0.50 (95% CI, 0.45–0.54) per 1000 person‐months; it was highest during the first month after the procedure (5.60 [95% CI, 4.89–6.42] per 1000 person‐months). Across the entire follow‐up period, the incidence rate was highest for people with CRT‐Ds (1.08 [95% CI, 0.81–1.44] per 1000 person‐months) and lowest for those with PPMs (0.42 [95% CI, 0.38–0.47] per 1000 person‐months) (Box 3).
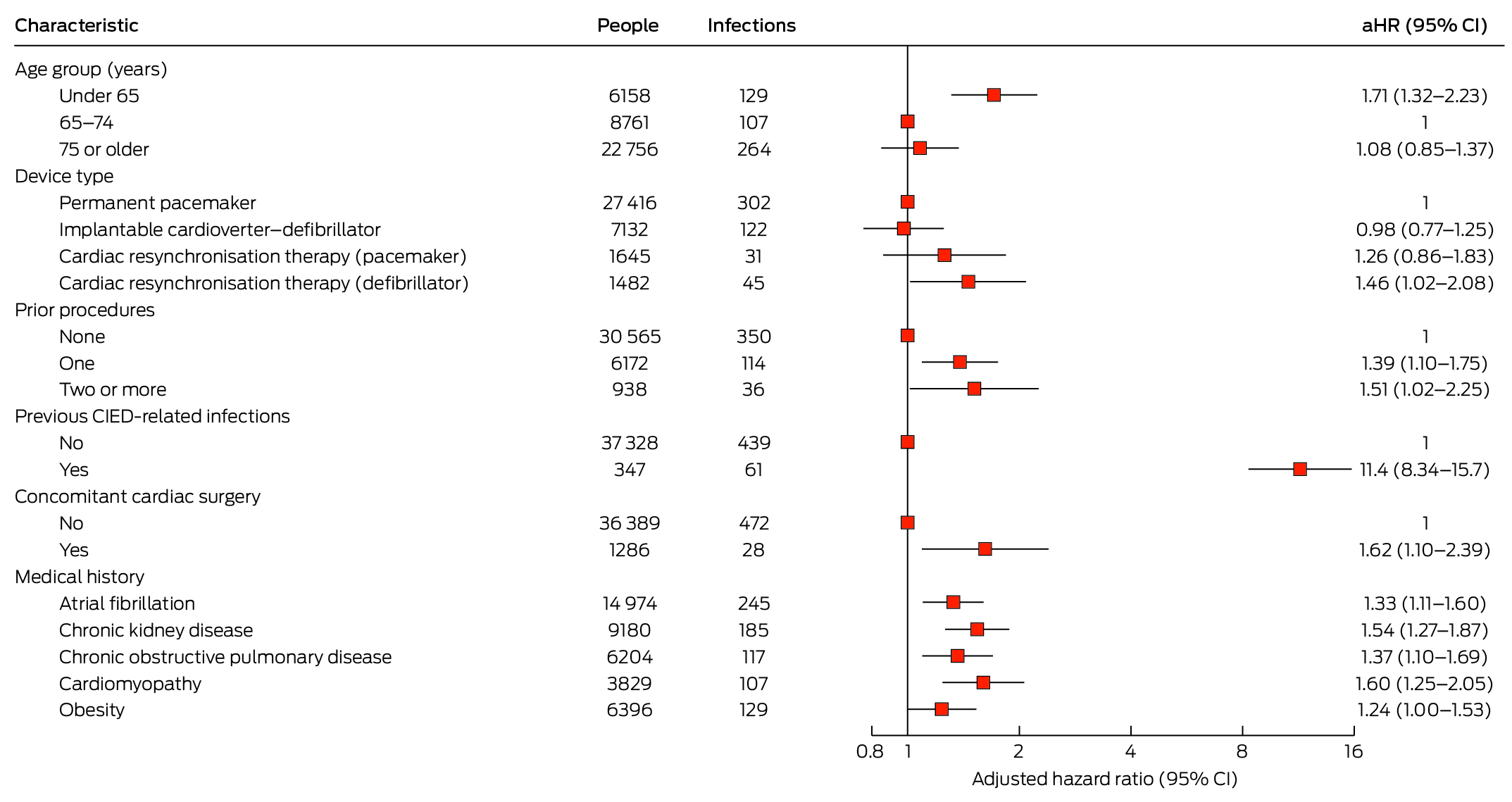
Factors associated with CIED‐related infections
Two variables significantly associated with CIED‐related infections in the minimally adjusted model (Supporting Information, table 5) were eliminated from the final Cox proportional hazards regression model: procedure type (highly correlated with number of prior procedures) and congestive heart failure. In the final model, the risk of CIED‐related infection was greater for people under 65 years of age than for those aged 65–74 years (aHR, 1.71; 95% CI, 1.32–2.23), for people with CRT‐D devices than for those with PPMs (aHR, 1.46; 95% CI, 1.02–2.08), for people who had previously undergone CIED procedures (two or more v no prior procedures: aHR, 1.51; 95% CI, 1.02–2.25) or had CIED‐related infections (aHR, 11.4; 95% CI, 8.34–15.7), for people who had undergone concomitant cardiac surgery (aHR, 1.62; 95% CI, 1.10–2.39), and for people with atrial fibrillation (aHR, 1.33; 95% CI, 1.11–1.60), chronic kidney disease (aHR, 1.54; 95% CI, 1.27–1.87), COPD (aHR, 1.37; 95% CI, 1.10–1.69), or cardiomyopathy (aHR 1.60; 95% CI, 1.25–2.05). The association with obesity was not statistically significant in the final model (aHR, 1.24; 95% CI, 0.9997–1.53) (Box 4).
Discussion
Our analysis of NSW hospital admissions data indicated that 1.1% of people who had CIED procedures during 2016–21 had CIED‐related infections within twelve months, and 1.3% by the end of follow‐up (median, 26 months). Among people at high risk of CIED‐related infections, as defined by the WRAP‐IT criteria, 1.5% experienced infection within twelve months of the procedure, and 2.5% by the end of follow‐up (median, 24.9 months). The overall infection rate was low (500 CIED‐related infections; 0.50 [95% CI, 0.45–0.54] per 1000 person‐months), but the risk of CIED‐related infection was greater for people under 65 years of age than for those aged 65–74 years, for people with CRT‐D devices than for those with PPMs, for people who had previously undergone CIED procedures or had CIED‐related infections, for those who had undergone concomitant cardiac surgery, and for people with atrial fibrillation, chronic kidney disease, COPD, or cardiomyopathy.
In a 2015 systematic review, the overall CIED infection rate in individual studies ranged between 0.7% and 4.3%.7 Population‐based studies similar to ours in Denmark17 and France18 respectively reported infection rates of 0.8% (six months) and 1.6% (36 months). However, factors such as device type and procedure type significantly influence the infection rate. For instance, 80% of devices in our study were PPMs and 74% of procedures were for new implants; in the Danish study, 71% of devices were PPMs and 74% new implants,17 while in the French study 84% of devices were PPMs and all procedures were for new implants.18 Two recently published large clinical trials, PADIT8 and WRAP‐IT,9 respectively found 12‐month CIED infection rates of 1.03% and 1.2%. Among people at high risk of CIED‐related infections (ie, excluding patients undergoing PPM procedures or first procedures other than for CRT‐D), the infection rate in our study was 1.5% over twelve months. The strict inclusion and exclusion criteria of the two large trials,8,9 and the fact that infection was the primary endpoint (potential Hawthorne effect: clinicians may have been more careful with infection control), may have influenced their infection rates.1
Other studies have also found that the risk of CIED‐related infections was lower in older people.6,19 The risk of infection declined with age in the PADIT trial;15 in a prospective study that included 46 000 Danish patients with pacemakers, the risk was much lower for people aged 80–89 years than for those aged 20–49 years (aHR, 0.29; 95% CI, 0.21–0.39).20 Although the biological reason for this difference is uncertain, it is clear that younger people with CIEDs are more likely to undergo multiple device procedures.15 We also found that having already had a CIED procedure or CIED‐related infection was associated with greater risk of infection after the index procedure. Further patient factors associated with infection in our study were chronic kidney disease, atrial fibrillation, COPD, and cardiomyopathy, consistent with systematic review findings of greater risk for patients with diabetes, chronic renal disease, COPD, atrial fibrillation, immunosuppression, or heart disease.6,7
In our fully adjusted model, the only major difference by device type was the greater risk of infection for people undergoing CRT‐D rather than PPM procedures, consistent with the findings of a meta‐analysis.7 However, the PADIT trial found that ICD, CRT‐P, and CRT‐D procedures were each associated with greater risks of infection than PPM procedures.15 CRT devices are larger than other CIEDs and require three leads and a longer procedure time,21 each of which could increase the risk of infection. In our minimally adjusted model, the risk was greater following CRT‐P than after PPM procedures. Similarly, the risk of infection was greater for revision and replacement procedures than for new insertions in our minimally adjusted model, but not in the fully adjusted model, presumably reflecting the collinearity of procedure type and prior procedure status; as it is therefore impossible to distinguish their effects, both procedure type and number of previous CIED procedures should be considered risk factors for CIED‐related infection.
Limitations
We relied on the accuracy of diagnosis and procedure codes in data collected for administrative purposes; we may have missed some CIED‐related infections, particularly prior to July 2017. However, the accuracy of administrative coding in Australia is high when compared with data in clinical registries: 85% agreement for the principal diagnosis and 80% for the principal procedure code.22 A sensitivity analysis of data from 1 July 2017 onwards yielded similar results to our main analysis (data not shown). The diagnostic codes used to identify CIED‐related infections could include conditions ranging from localised pocket infections to endocarditis, bacteraemia, and septic shock. We did not have information on peri‐operative management of CIED‐related infections, such as antibiotic use. The impact of some patient‐level covariates included in the PADIT score,15 such as immunosuppression and long term or recent high‐dose steroid use, could not be assessed. We identified medical conditions recorded during the index admission as well as during hospital admissions in the preceding twelve months. This approach may have missed or misclassified some conditions, which would have weakened the reported associations with CIED‐related infection. Finally, residual confounding by unmeasured factors is possible because of the observational study design.
Conclusions
The overall CIED‐related infection rate was low, but was higher for people defined by recent clinical trials as being at greater risk of such infection. We identified several patient, device, and procedural factors associated with greater risk of CIED‐related infection. Knowledge of these factors can help clinicians discuss infection risk with individual patients, identify those at particular risk of infection, and inform decisions about device type, upgrades and replacements, and prophylactic interventions. Despite uncertainty regarding the effectiveness of peri‐procedural antibiotic prophylaxis,2,8 evidence from the WRAP‐IT trial suggests that an absorbable, antibiotic‐eluting envelope can prevent CIED‐related infection, and their use has recently been recommended by the European Heart Rhythm Association.9
Box 1 – Characteristics of people who underwent cardiac implantable electronic device (CIED) procedures in New South Wales, 2016–21 (public hospitals) or 2016–20 (private hospitals), by CIED infection status*
|
Characteristic |
No CIED‐related infection |
CIED‐related infection |
P |
||||||||||||
|
|
|||||||||||||||
|
Number of patients |
37 175 (98.7%) |
500 (1.3%) |
|
||||||||||||
|
Age (years), mean (standard deviation) |
75.8 (12.8) |
72.2 (15.2) |
< 0.001 |
||||||||||||
|
Sex (female) |
13 589 (36.6%) |
172 (34.4%) |
0.32 |
||||||||||||
|
Device type |
|
|
< 0.001 |
||||||||||||
|
Permanent pacemaker |
27 114 (72.9%) |
302 (60.4%) |
|
||||||||||||
|
Implantable cardioverter–defibrillator |
7010 (18.9%) |
122 (24.4%) |
|
||||||||||||
|
Cardiac resynchronisation therapy with pacemaker |
1614 (4.3%) |
31 (6.2%) |
|
||||||||||||
|
Cardiac resynchronisation therapy with defibrillator |
1437 (3.9%) |
45 (9.0%) |
|
||||||||||||
|
Procedure type |
|
|
< 0.001 |
||||||||||||
|
New |
27 325 (73.5%) |
328 (65.6%) |
|
||||||||||||
|
Revision |
588 (1.6%) |
15 (3.0%) |
|
||||||||||||
|
Replacement (including upgrades) |
9262 (24.9%) |
157 (31.4%) |
|
||||||||||||
|
Prior CIED procedures† |
|
|
< 0.001 |
||||||||||||
|
None |
30 215 (81.3%) |
350 (70.0%) |
|
||||||||||||
|
One |
6058 (16.3%) |
114 (22.8%) |
|
||||||||||||
|
Two or more |
902 (2.4%) |
36 (7.2%) |
|
||||||||||||
|
Previous CIED‐related infections† |
286 (0.8%) |
61 (12.2%) |
< 0.001 |
||||||||||||
|
Emergency admission |
11 488 (30.9%) |
153 (30.6%) |
0.88 |
||||||||||||
|
Concomitant cardiac procedure |
1258 (3.4%) |
28 (5.6%) |
0.007 |
||||||||||||
|
Private insurance |
20 539 (55.2%) |
261 (52.2%) |
0.17 |
||||||||||||
|
Length of stay (days), median (interquartile range) |
2 (1–6) |
3 (1–11) |
< 0.001 |
||||||||||||
|
Medical history‡ |
|
|
|
||||||||||||
|
Diabetes |
11201 (30.1%) |
168 (33.6%) |
0.09 |
||||||||||||
|
Hypertension |
28 777 (77.4%) |
393 (78.6%) |
0.53 |
||||||||||||
|
Coronary artery disease |
20 089 (54.0%) |
311 (62.2%) |
< 0.001 |
||||||||||||
|
Congestive heart failure |
14 551 (39.1%) |
288 (57.6%) |
< 0.001 |
||||||||||||
|
Atrial fibrillation |
14 729 (39.6%) |
245 (49.0%) |
< 0.001 |
||||||||||||
|
Cardiomyopathy |
3722 (10.0%) |
107 (21.4%) |
< 0.001 |
||||||||||||
|
Peripheral vascular disease |
9165 (24.7%) |
182 (36.4%) |
< 0.001 |
||||||||||||
|
Stroke |
3499 (9.4%) |
61 (12.2%) |
0.034 |
||||||||||||
|
Chronic obstructive pulmonary disease |
6087 (16.4%) |
117 (23.4%) |
< 0.001 |
||||||||||||
|
Sleep apnoea |
1355 (3.6%) |
36 (7.2%) |
< 0.001 |
||||||||||||
|
Chronic kidney disease |
8995 (24.2%) |
185 (37.0%) |
< 0.001 |
||||||||||||
|
Obesity |
6267 (16.9%) |
129 (25.8%) |
< 0.001 |
||||||||||||
|
Cardiac surgery history‡ |
|
|
|
||||||||||||
|
Coronary artery bypass graft |
609 (1.6%) |
12 (2.4%) |
0.18 |
||||||||||||
|
Valve surgery |
900 (2.4%) |
23 (4.6%) |
0.002 |
||||||||||||
|
Hospital type |
|
|
0.27 |
||||||||||||
|
Public |
20 862 (56.1%) |
293 (58.6%) |
|
||||||||||||
|
Private |
16 313 (43.9%) |
207 (41.4%) |
|
||||||||||||
|
|
|||||||||||||||
|
* The characteristics of patients at high risk of CIED‐related infections are included in the Supporting Information, table 4. † From 1 July 2001 to the index CIED procedure. ‡ As recorded in hospital records for index admission and all hospital admissions in the twelve months preceding the index admission. |
|||||||||||||||
Box 2 – Cumulative incidence of first cardiac implantable electronic device (CIED)‐related infections, by device type (A) and (procedure type (B): Kaplan–Meier curves
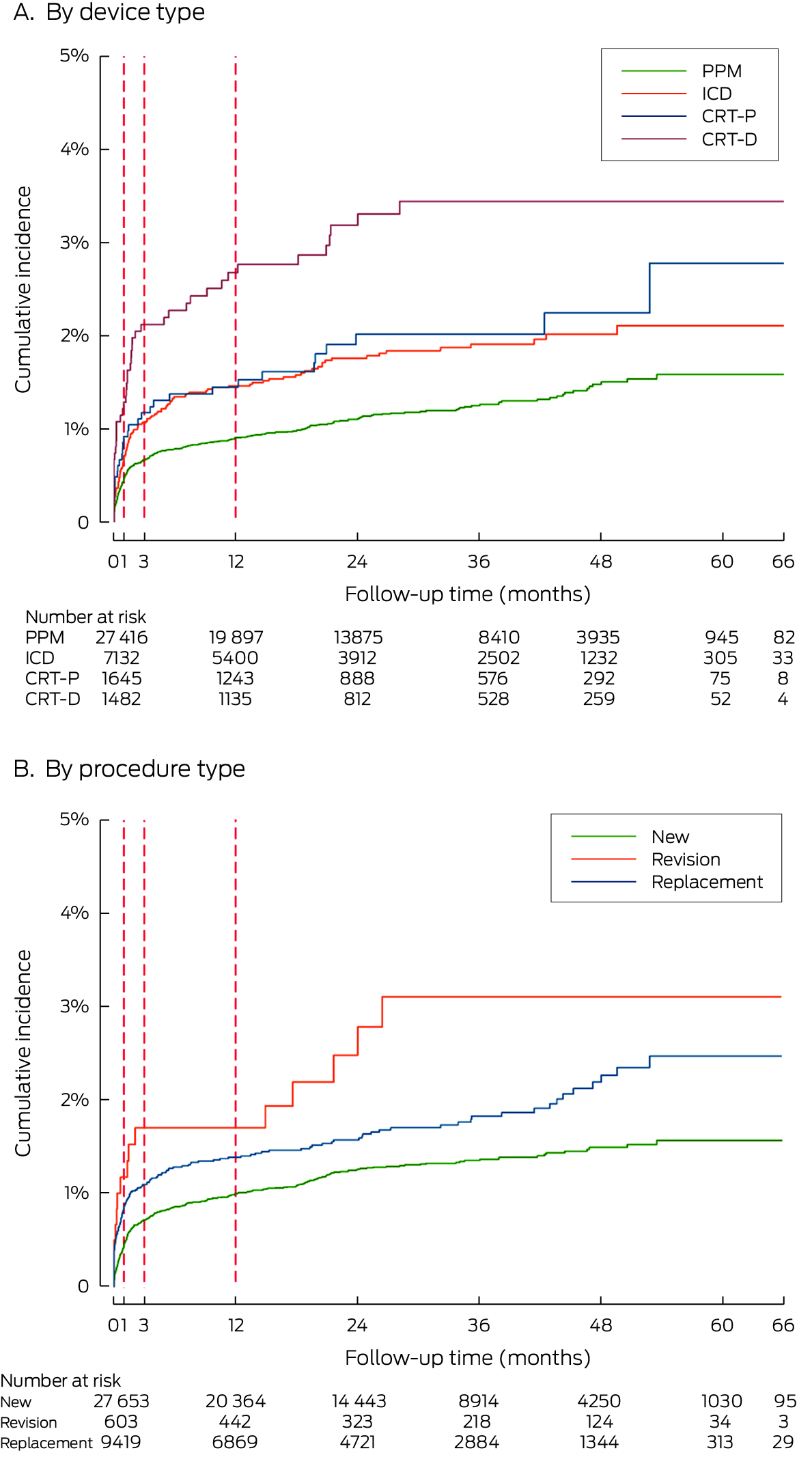
CRT‐D = cardiac resynchronisation therapy (defibrillator); CRT‐P = cardiac resynchronisation therapy (pacemaker), ICD = implantable cardioverter–defibrillator; PPM = permanent pacemaker.
Box 3 – Incidence of cardiac implantable electronic device (CIED)‐related infections, by time since CIED procedure, overall and by device type
|
Device type/follow‐up time |
Person‐months |
Infections |
Incidence rate per 1000 person‐months (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
All procedure types (37 675 people) |
|
|
|
||||||||||||
|
0–1 month |
37 137 |
208 |
5.60 (4.89–6.42) |
||||||||||||
|
2–3 months |
71 456 |
98 |
1.37 (1.13–1.67) |
||||||||||||
|
4–12 months |
280 924 |
91 |
0.32 (0.26–0.40) |
||||||||||||
|
13 or more months |
617 211 |
103 |
0.17 (0.14–0.20) |
||||||||||||
|
End of follow‐up |
1 006 728 |
500 |
0.50 (0.45–0.54) |
||||||||||||
|
Permanent pacemaker (27 416 people) |
|
|
|
||||||||||||
|
0–1 month |
27 032 |
125 |
4.62 (3.88–5.51) |
||||||||||||
|
2–3 months |
51 981 |
56 |
1.08 (0.83–1.40) |
||||||||||||
|
4–12 months |
203 118 |
55 |
0.27 (0.21–0.35) |
||||||||||||
|
13 or more months |
436 058 |
66 |
0.15 (0.12–0.19) |
||||||||||||
|
End of follow‐up |
718 189 |
302 |
0.42 (0.38–0.47) |
||||||||||||
|
Implantable cardioverter–defibrillator (7132 people) |
|
|
|
||||||||||||
|
0–1 month |
7036 |
49 |
6.96 (5.26–9.21) |
||||||||||||
|
2–3 months |
13 586 |
26 |
1.91 (1.30–2.81) |
||||||||||||
|
4–12 months |
54 215 |
25 |
0.46 (0.31–0.68) |
||||||||||||
|
13 or more months |
125 827 |
22 |
0.17 (0.12–0.27) |
||||||||||||
|
End of follow‐up |
200 664 |
122 |
0.61 (0.51–0.73) |
||||||||||||
|
Cardiac resynchronisation therapy (pacemaker) (1645 people) |
|
|
|
||||||||||||
|
0–1 month |
1617 |
15 |
9.28 (5.59–15.4) |
||||||||||||
|
2–3 months |
3091 |
< 5 |
NA |
||||||||||||
|
4–12 months |
12 421 |
< 5 |
NA |
||||||||||||
|
13 or more months |
29 058 |
8 |
0.28 (0.14–0.55) |
||||||||||||
|
End of follow‐up |
46 187 |
31 |
0.67 (0.47–0.95) |
||||||||||||
|
Cardiac resynchronisation therapy (defibrillator) (1482 people) |
|
|
|
||||||||||||
|
0–1 month |
1452 |
19 |
13.1 (8.35–20.5) |
||||||||||||
|
2–3 months |
2798 |
12 |
4.29 (2.44–7.55) |
||||||||||||
|
4–12 months |
11 171 |
7 |
0.63 (0.30–1.31) |
||||||||||||
|
13 or more months |
26 267 |
7 |
0.27 (0.13–0.56) |
||||||||||||
|
End of follow‐up |
41 688 |
45 |
1.08 (0.81–1.44) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; NA = not available (suppressed raw count numbers). |
|||||||||||||||
Received 31 March 2023, accepted 22 January 2024
- Md Shajedur Rahman Shawon1
- Oluwadamisola T Sotade1
- Joan Li2
- Michelle D Hill3
- Liesl Strachan3
- Gabrielle Challis3
- Kate King3
- Sze‐Yuan Ooi2
- Louisa Jorm1
- 1 Centre for Big Data Research in Health, University of New South Wales, Sydney, NSW
- 2 Prince of Wales Hospital, Sydney, NSW
- 3 Medtronic Australasia Pty Ltd, Sydney, NSW
Open access:
Open access publishing facilitated by the University of New South Wales, as part of the Wiley – University of New South Wales agreement via the Council of Australian University Librarians.
Data sharing:
The data underlying this article will be shared on reasonable request to the corresponding author.
This study was funded by Medtronic Australasia Pty Ltd through funding to the Centre for Big Data Research in Health, University of New South Wales.
The dataset includes data related to devices manufactured by Medtronic and other companies that supply such devices in Australia and New Zealand. The Medtronic Australasia authors (Michelle Hill, Liesl Strachan, Gabrielle Challis, Kate King) contributed to study conception and design, interpreting data, critically reviewing the manuscript, and approving the manuscript. They had no access to the raw study data and did not participate in the statistical analysis.
- 1. Wilkoff BL, Boriani G, Mittal S, et al; WRAP‐IT Investigators. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP‐IT trial. Circ Arrhythm Electrophysiol 2020; 13: e008280.
- 2. Blomström‐Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2020; 41: 2012‐2032.
- 3. Sohail MR, Henrikson CA, Braid‐Forbes MJ, et al. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011; 171: 1821‐1828.
- 4. Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter–defibrillator infections. J Am Coll Cardiol 2007; 49: 1851‐1859.
- 5. Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care and Outcomes Research. A summary of the update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. J Am Dent Assoc 2011; 142: 159‐165.
- 6. Han HC, Hawkins NM, Pearman CM, et al. Epidemiology of cardiac implantable electronic device infections: incidence and risk factors. Europace 2021; 23: iv3‐iv10.
- 7. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta‐analysis. Europace 2015; 17: 767‐777.
- 8. Krahn AD, Longtin Y, Philippon F, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018; 72: 3098‐3109.
- 9. Tarakji KG, Mittal S, Kennergren C, et al; WRAP‐IT Investigators. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019; 380: 1895‐1905.
- 10. Mittal S, Tsiouris SJ. Patients at high risk for CIED infection: does our perception mirror reality? J Am Coll Cardiol 2019; 74: 2855‐2857.
- 11. Roder C, Gunjaca V, Otome O, et al. Cost and outcomes of implantable cardiac electronic device infections in Victoria, Australia. Heart Lung Circ 2020; 29: e140‐e146.
- 12. Ganesan AN, Moore K, Horton D, Heddle W, et al. Complications of cardiac implantable electronic device placement in public and private hospitals. Intern Med J 2020; 50: 1207‐1216.
- 13. Moore K, Ganesan A, Labrosciano C, et al. Sex differences in acute complications of cardiac implantable electronic devices: implications for patient safety. J Am Heart Assoc 2019; 8: e010869.
- 14. Independent Health and Aged Care Pricing Authority. ICD‐10‐AM/ACHI/ACS. https://www.ihacpa.gov.au/health‐care/classification/icd‐10‐amachiacs (viewed Apr 2024).
- 15. Birnie DH, Wang J, Alings M, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019; 74: 2845‐2854.
- 16. Hosmer DW, Lemeshow S. Model‐building strategies and methods for logistic regression. In: Hosmer DW, Lemeshow S. Applied logistic regression, second edition. Hoboken: Wiley, 2000; pp. 91‐142.
- 17. Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: 1186‐1194.
- 18. Clémenty N, Carion PL, de Léotoing L, et al. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace 2018; 20: 1974‐1980.
- 19. Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace 2010; 12: 64‐70.
- 20. Johansen JB, Jørgensen OD, Møller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population‐based cohort study of 46 299 consecutive patients. Eur Heart J 2011; 32: 991‐998.
- 21. European Heart Rhythm Association (EHRA); European Society of Cardiology (ESC); Heart Rhythm Society; Heart Failure Society of America (HFSA); American Society of Echocardiography (ASE); American Heart Association (AHA); European Association of Echocardiography (EAE) of ESC; Heart Failure Association of ESC (HFA); Daubert JC, Saxon L, Adamson PB, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow‐up recommendations and management. Europace 2012; 14: 1236‐1286.
- 22. Henderson T, Shepheard J, Sundararajan V. Quality of diagnosis and procedure coding in ICD‐10 administrative data. Med Care 2006; 44: 1011‐1019.






Abstract
Objectives: To quantify the rate of cardiac implantable electronic device (CIED)‐related infections and to identify risk factors for such infections.
Design: Retrospective cohort study; analysis of linked hospital admissions and mortality data.
Setting, participants: All adults who underwent CIED procedures in New South Wales between 1 January 2016 and 30 June 2021 (public hospitals) or 30 June 2020 (private hospitals).
Main outcome measures: Proportions of patients hospitalised with CIED‐related infections (identified by hospital record diagnosis codes); risk of CIED‐related infection by patient, device, and procedural factors.
Results: Of 37 675 CIED procedures (23 194 men, 63.5%), 500 were followed by CIED‐related infections (median follow‐up, 24.9 months; interquartile range, 11.2–40.8 months), including 397 people (1.1%) within twelve months of their procedures, and 186 of 10 540 people (2.5%) at high risk of such infections (replacement or upgrade procedures; new cardiac resynchronisation therapy with defibrillator, CRT‐D). The overall infection rate was 0.50 (95% confidence interval [CI], 0.45–0.54) per 1000 person‐months; it was highest during the first month after the procedure (5.60 [95% CI, 4.89–6.42] per 1000 person‐months). The risk of CIED‐related infection was greater for people under 65 years of age than for those aged 65–74 years (adjusted hazard ratio [aHR], 1.71; 95% CI, 1.32–2.23), for people with CRT‐D devices than for those with permanent pacemakers (aHR, 1.46; 95% CI, 1.02–2.08), for people who had previously undergone CIED procedures (two or morev none: aHR, 1.51; 95% CI, 1.02–2.25) or had CIED‐related infections (aHR, 11.4; 95% CI, 8.34–15.7), or had undergone concomitant cardiac surgery (aHR, 1.62; 95% CI, 1.10–2.39), and for people with atrial fibrillation (aHR, 1.33; 95% CI, 1.11–1.60), chronic kidney disease (aHR, 1.54; 95% CI, 1.27–1.87), chronic obstructive pulmonary disease (aHR, 1.37; 95% CI, 1.10–1.69), or cardiomyopathy (aHR 1.60; 95% CI, 1.25–2.05).
Conclusions: Knowledge of risk factors for CIED‐related infections can help clinicians discuss them with their patients, identify people at particular risk, and inform decisions about device type, upgrades and replacements, and prophylactic interventions.