Medicines are the most frequent health care intervention type. Their safe use provides significant benefits, but they are also associated with higher rates of errors and adverse events than other interventions.1 Reasons for medication-related problems include consulting time constraints for doctors and pharmacists, the involvement of multiple prescribers and dispensers in patient care, increased complexity of care and reliance on medicines to manage chronic illness, suboptimal recording of information in clinical records and sharing of this information, and the lack of financial incentives and staff for identifying, averting, and rectifying medication-related problems.2,3,4 They also arise because of incomplete adherence to prescribed medication use, confusion regarding treatment regimens, over- and under-prescribing, and adverse drug interactions.5,6
Serious medication-related problems are defined as those that can lead to unplanned hospitalisations and health sequelae, including serious complications and death.7 In Australia, an estimated 400 000 emergency presentations and 250 000 hospital admissions are related to potentially preventable medication-related problems each year, at a cost to the healthcare system of more than $1.4 billion.7,8
Two publicly funded primary care approaches could reduce medication-related problems in Australia: payments to pharmacists for providing medicine counselling when dispensing of Pharmaceutical Benefits Scheme (PBS) medicines; and periodic medicine management services, such as MedsCheck, Diabetes MedsCheck, and home medicines reviews.9 While structured medicine reviews can proactively identify medication-related problems, these services have little effect on those that lead to potentially preventable hospitalisations.10,11 The untimeliness of reviews, caps on how many can be provided,9 and a lack of accredited pharmacists in some locations may partially explain this lack of effectiveness.4 Further, pharmacists conducting home medicines reviews may focus more on patient confusion about the medicine regimen and similar factors, and may not have access to up-to-date clinical records.
Innovative systemic approaches to identifying and rectifying medicines safety problems in primary care, supported by digital health technology and new remuneration models, are needed to address their causes. In the United Kingdom, pharmacist-led interventions including electronic dashboards, education and benchmarking for general practitioners, and financial incentives in primary care significantly reduce the frequency of high risk prescribing.12,13,14,15
ACTMed is a whole-of-system approach that defines medicines safety as a proactive and team-based collaboration between people attending general practices, pharmacists, general practitioners, and other health care providers. ACTMed is a quality improvement activity based on innovative digital technologies and financial incentives for encouraging pharmacists to work with general practitioners in mainstream general practices and in Aboriginal and Torres Strait Islander community-controlled health organisations. Its aim is to reduce the risk of medicine-related harm, reduce health care costs and improve its efficiency, and to enhance person-centred care.
Aim of the study
The aim of the ACTMed (ACTivating primary care for MEDicine safety) trial, the first of this type of intervention in Australia, is to evaluate the effectiveness and cost-effectiveness of a general practice-based intervention for reducing the risk of serious medication-related problems, and its impact on health care costs, health care efficiency and coordination, and patients’ experience of care. For the purposes of this study, we will restrict our analysis to serious medication-related problems, pre-specified by five clinical indicators that identify under-prescribing of condition-specific medications or monitoring tests.
Timetable and participating sites
- Design phase: 1 May 2022 – 31 March 2023.
- Pilot test phase (three primary practices, including one Aboriginal and Torres Strait Islander Community Controlled Health Organisation): 1 April 2023 – 31 July 2023.
- Commence practice recruitment for main trial: 22 April 2023.
- Complete practice recruitment: 31 July 2023.
- Commence data collection: 1 September 2023.
- Complete primary outcome data collection: 31 March 2024.
- Complete secondary outcomes data collection: 30 August 2024.
The list of participating practices who have given consent to be named will be available at the completion of the study on application to the corresponding author.
Methods
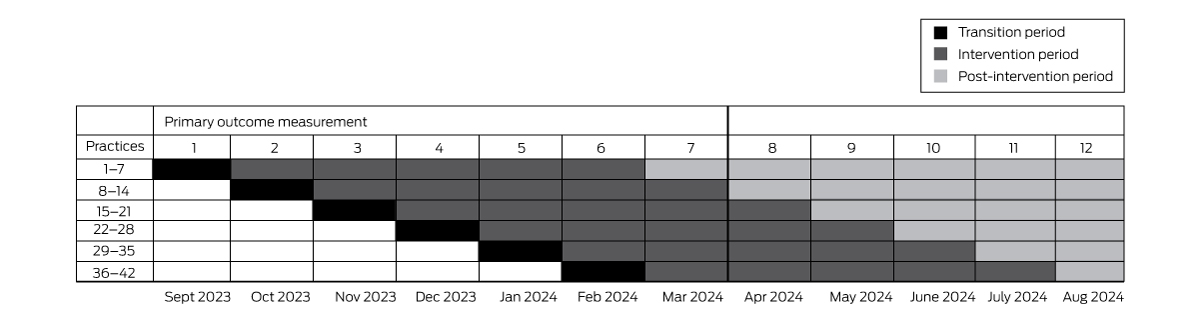
The effectiveness of the ACTMed intervention will be assessed in a stepped wedge cluster randomised trial. This trial type is a pragmatic alternative to a parallel group cluster randomised trial when it would be inefficient to simultaneously commence the intervention at all participating sites, or if there are ethical or recruitment and attrition concerns regarding control group participants not receiving the intervention.16 Blinding of the practices and the research team to the intervention will not be possible. Practices will be randomly allocated to intervention starting dates that will be communicated to them with sufficient notice for planning their participation.
The trial will be undertaken at each participating practice over a period of six months: a one month transition period for training, induction of practice pharmacists (when required), and software troubleshooting, and five months of intervention data collection. After seven months, all 42 practices will have commenced the intervention phase; data collection for the primary outcome will cease one month after the final practice has implemented the intervention (Box 1). Data will be collected during the subsequent five months for the major secondary outcome (persistence of any ACTMed intervention effect). The trial design allows all practices to receive funding for a pharmacist for six intervention months, although not all practices will contribute equal intervention time to the primary outcome measurement; this approach reduces barriers to practice recruitment.
Software for ethical data acquisition (GRHANITE, University of Melbourne; http://grhanite.unimelb.edu.au/technologies) and clinical decision support software (Future Health Today; purpose-built for general practice quality improvement activities: https://futurehealthtoday.com.au) will be installed at each practice in advance but not activated until the intervention starts. GRHANITE can produce summary baseline data analyses from historical general practice data.
Practice eligibility and recruitment
We recruited practices for both phases of the study through the Brisbane South Primary Health Network and the National Aboriginal Community Controlled Health Organisation, using their established communication channels and networks. At least one Aboriginal Community Controlled Health Organisation (ACCHO) will also be included to ensure that ACTMed is designed and evaluated without barriers that could preclude future implementation in community-controlled health services. The eligibility criteria for practice participation are:
- The practice must have a minimum of 5000 active adult patients (at least two consultations during the past three years), and be paired with at least one Home Medicines Review-accredited pharmacist working in a local community pharmacy or general practice. The size criterion will not be applied to up to six general practices and smaller ACCHOs included for equity of access reasons, nor to practices with culturally and linguistically diverse clienteles.
- The practice must currently use Medical Director or Best Practice software, each of which is compatible with GRHANITE and Future Health Today, and its information technology system must satisfy the requirements for installing GRHANITE and Future Health Today.
- The practice has the capacity to participate in the trial, and consents to providing de-identified individual-level and aggregate practice level data relevant to the primary and secondary outcomes according to the service agreement with the University of Queensland.
Service agreements will be negotiated by the practices, the ACCHOs, the University of Queensland, and the University of Melbourne. Pharmacist recruitment will be supported by the Pharmaceutical Society of Australia.
The ACTMed intervention (Box 2)
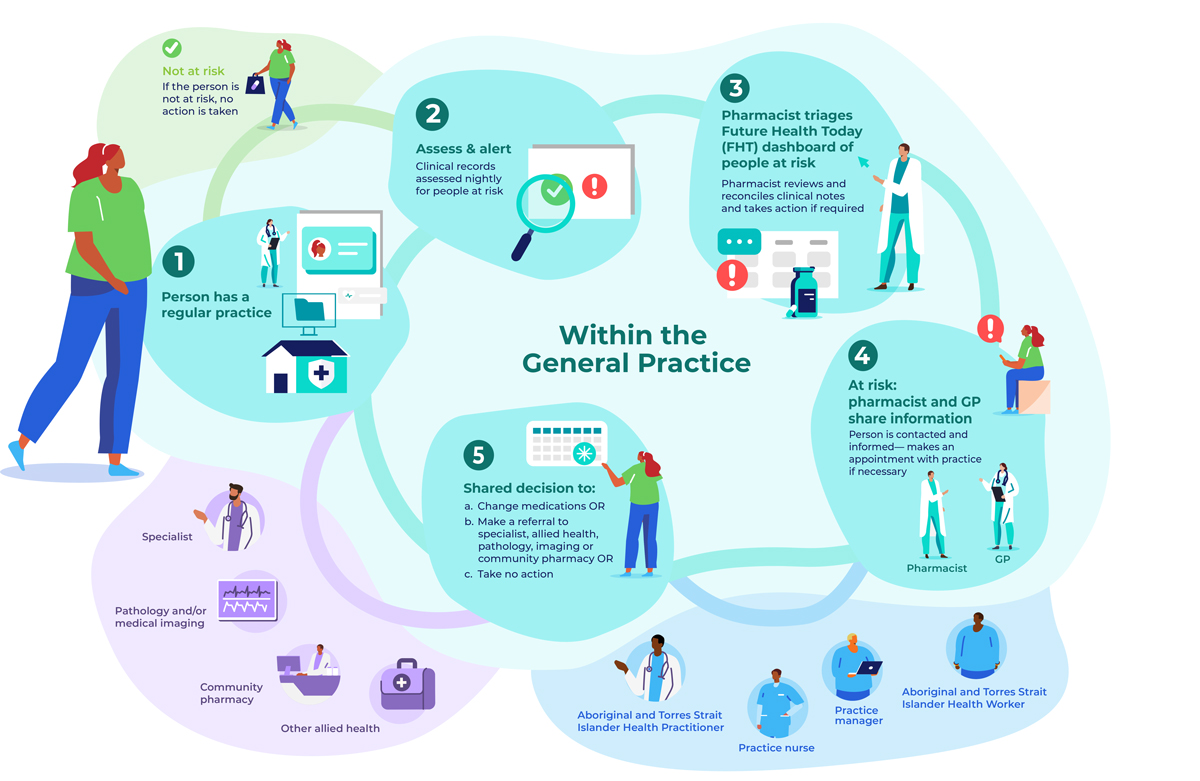
A practice pharmacist, already working in the practice or recruited for the trial, will use GRHANITE and Future Health Today (Supporting Information, part 2) to identify adults (18 years or older) at risk of serious medication-related problems. Each night, Future Health Today clinical indicator algorithms applied to the clinical databases of participating practices will generate lists of people potentially at risk. The trial pharmacist will use the Future Health Today dashboard to screen the clinical records of identified patients and compare them with dispensing information in My Health Record and other practice clinical records to generate a consolidated list of patients at risk to be triaged, and to remove any people incorrectly classified as being at risk. When My Health Record information is not available, the pharmacist will apply a medicines reconciliation approach.17
During triage, the pharmacist will establish the type of medication-related problem, the action required, and whether the patient has any cultural or health needs that require notification of a particular health practitioner involved in their care. Depending on the type of risk, actions could include contacting the person directly, or contacting their usual community pharmacist for the purpose of medication reconciliation. Other actions could be asking people to visit their general practitioner or pharmacist for review, arranging pathology tests or medical imaging, and referral to a specialist or allied health professional. If action is yet to be taken, a flag (point-of-care pop-up warning) will appear in the person's medical record. Guideline-based clinical recommendations for general practitioners and pharmacists are provided by Future Health Today, and it can also be used to record actions taken.
ACTMed will be implemented in a quality improvement framework, developed and implemented by the Brisbane South Primary Health Network,18 to ensure standardisation across practices. Practices will be compensated for their participation and provided with funding for the in-practice pharmacist. Benchmarking reports on clinical indicators (number of people identified as being at risk as a proportion of patients at risk, as defined below) will be generated each month from aggregated Future Health Today data managed by the research team, and reported to all practices where the intervention has commenced. Additionally, the trial pharmacist will be encouraged to deliver feedback to general practitioners at face-to-face and practice meetings, and by dissemination of dashboard results.
Training
Pharmacists, general practitioners, and practice support staff, including practice managers and nurses, will be trained in the ACTMed intervention, including:
- an activity-based quality improvement module about the ACTMed process, including an evidence-based summary for each clinical indicator (one hour; online, self-directed);
- a Future Health Today training session by the University of Melbourne Future Health Today team (one hour);
- a group quality improvement session for pharmacists on how to lead and document the quality improvement cycle in accordance with the primary health network framework and in a manner eligible for Practice Incentives Program payments (one hour; online forum led by the Brisbane South Primary Health Network quality improvement team);
- a community of practice session run by an experienced clinical pharmacist to enhance peer-to-peer learning, including information on improving socialisation of the ACTMed service, quality improvement activities, data collection, and review, as well as clinical information (1.5 hours every two months; minimum of three sessions per six months of trial involvement); and,
- for pharmacists with no or little experience of working in a general practice: two days of onsite training at a mentor's practice and on-call support from the mentor as required.
Clinical indicators: people at risk of serious medication-related problems
Each clinical indicator has two parts: the definition for identifying people at risk of medication-related problems so that appropriate action can be undertaken in primary care; and the reason for the potentially preventable medication-related hospitalisation outcome.
The incidence or prevalence of clinical indicators based on our findings in earlier studies19,20,21 were estimated using a tranche of Data for Decisions [Patron] data (Supporting Information, part 2).22 Further criteria for selecting clinical indicators were prevalence rates, the possibility of incorrect diagnoses, the availability of interventions that could be undertaken by qualified pharmacists and general practitioners, and other practical considerations. We applied a modified nominal group technique23 to rank the clinical indicators; the group included four general practitioners and four pharmacists recruited from the authors’ professional networks, including the Future Health Today general practice advisory group. The research team and ACTMed steering committee selected the four top ranked indicators and one further indicator (ranked number seven) for the ACTMed trial; the fifth and sixth ranked indicators were not used because of problems with developing robust algorithms (Box 3).
Outcomes
The primary outcome will be the proportion of people at risk of serious medication-related problems who experience such problems; we will compare the proportions during the pre-intervention and intervention periods (patient-level analysis). For the key secondary outcome, we will compare these proportions in a practice-level analysis. Other secondary outcomes will be changes in the rates of potentially preventable medication-related hospitalisations and deaths, changes in medication-related problems rates beyond the trial period, patients’ views of the ACTMed service, changes in job satisfaction among participating health care practitioners, and the rates of incorrect identification of people as being at risk, by clinical indicator (Box 4).
Sample size
We estimated that 42 participating practices would be required to achieve 90% power for detecting a 10% practice-level difference in medication-related problem rates between the pre-intervention and intervention periods (a = 0.05, inter-cluster correlation = 0.05) in the primary outcome, assuming the baseline medication-related problem rate among people at risk is 0.08, and that six practices will not complete the trial and therefore not deliver data.
For the key secondary outcome (difference in medication-related problem rates at the practice level), the sample size will be sufficient to achieve 80% statistical power for detecting a 19% difference in medication-related problem rates between the pre-intervention and intervention periods. The anticipated difference was informed by the proportion of people at risk who had medication-related problems derived from an analysis of Patron data for 75 Victorian general practices (Supporting Information, part 2).
We anticipate a total of 35 238 people at risk, based on an estimated 839 patients per practice across all indicators. This is comparable with estimates of the at-risk population and effect sizes of British intervention trials;12,13,14 one group that examined individual-level time series data13,14 found a 40% reduction in high-risk prescribing, but a later national study found an estimated 25% reduction at the more conservative practice level.26 We used the Stata 17 steppedwedge command for sample size calculations.27
Statistical analysis
We will undertake intention-to-treat (ITT) analyses of outcomes data. For the primary outcome, we will use a mixed effects logistic regression model. Fixed effects will include ACTMed exposure (intervention, pre-intervention) and time; random effects will account for clustering (at the practice level) and repeated measures (at the patient level). The fixed effect time will account for time-dependent changes that may affect the primary outcome, such as change in general practitioner prescribing behaviour. A supplementary analysis at the practice level will use mixed effects models to account for the effects of clustering and repeated measures over time. The statistical analysis plan is included in the clinical trial registration.
Economic evaluation
We will estimate the cost per averted serious medication-related problem and the cost per averted potentially preventable medication-related hospitalisation in an in-trial and modelled economic evaluation. In addition, the cost savings associated with lower numbers of potentially preventable medication-related hospitalisations and emergency department visits will be estimated on the basis of Queensland hospitals registry data. The savings will be extrapolated to estimate the benefits were ACTMed introduced across Australia, and the cost of doing so will be estimated by extrapolating the costs associated with its implementation during the trial.
Data linkage
Linkage of Queensland Health hospitalisations and Queensland deaths registry data, accessed under a Public Health Act application, will preserve the privacy of patients in accordance with privacy standards for general practice data.28 We will request data for all emergency department and inpatient care episodes with International Classification of Diseases (ICD-10) codes29 corresponding to the trial clinical indicators, from six months before the start of the trial to six months after the final practice implements the intervention.
Ethics approval
The University of Queensland Human Research Ethics Committee approved the pilot (2021/HE002189) and trial phases of the ACTMed study (2022/HE002136). Access to Patron data was granted by the Patron Data Governance Committee (PAT052ACTMed). Access to linked hospitalisations and deaths data are subject to a Public Health Act application (pending).
Consent and potential harms
Consistent with National Health and Medical Research Council (NHMRC) guidance,30 the requirement for individual patient consent to use of their health-related data was waived by the University of Melbourne human research ethics committee (reference, 1647396) because the risk to patients is minimal, a transparent data governance framework has been developed,31 and obtaining explicit consent from each patient would be both impractical and likely to influence trial outcomes. The use of GRHANITE to extract de-identified practice data for ACTMed evaluation is deemed to pose only a low risk under NHMRC guidelines. Participating general practices will sign legal agreements regarding data sharing, and will display in their waiting rooms information about the ACTMed intervention and the sharing of de-identified data. If a person advises a general practice that they do not want their data to be shared, this denial of consent will be recorded by the practice in GRHANITE, and no data will be extracted for that patient.
Data safety monitoring
The intervention consists of registered pharmacists and general practitioners revising the use of registered medicines according to established therapeutic and treatment guidelines; the risk to patient safety is consequently low. A data safety monitoring board will not be established, consistent with NHMRC guidance for low or negligible risk interventions; the ACTMed steering committee will be responsible for data safety monitoring.
Dissemination of findings
The first Australian trial to develop and test an integrated, proactive, and targeted medicines safety service for primary care will provide information about the effectiveness and cost of a digital strategy for averting medication-related problems. A comprehensive dissemination plan will be developed by the research team, the steering committee, the consumer advisory group, project partners, and representatives from some participating practices. Aboriginal and Torres Strait Islander communities will be supported in designing and leading meaningful community-level dissemination in formats of their choice. We will report our findings in trial reports, at national and overseas conferences and scientific meetings, and in peer-reviewed journal articles. All study data will be managed and stored according to the University of Queensland research data management policy, using the secure University of Queensland Research Data Manager.
Trial registration
The pilot (ACTRN12622000595718; 21 April 2022) and full ACTMed trials (ACTRN12622000574741; 14 April 2022; updated 7 August 2023) were prospectively registered with the Australian New Zealand Clinical Trials Registry.
Author contributions
All listed authors contributed to the development and revision of this protocol, made significant contributions to its intellectual content, and approved the final version. Jean Spinks and Lisa Nissen led project development. Douglas Boyle led software development. Kerry Hall provided cultural expertise for engagement and co-design activities with Aboriginal and Torres Strait Islander people, communities, and community-controlled health services.
Funding statement
The ACTMed trial is funded by the Australian government through the Medical Research Future Fund (MRFQ1000023).
Box 3 – The ACTMed trial: clinical indicators
|
Indicator |
At-risk population (denominator) |
At-risk population with medication-related problem (numerator) |
Event leading to potentially preventable medication-related hospitalisation |
||||||||||||
|
|
|||||||||||||||
|
Atrial fibrillation |
People with atrial fibrillation diagnosis AND[No diagnosis of stroke or transient ischaemic attack AND CHA2DS2-VA24 score = 1] OR[History of stroke or transient ischaemic attack] |
Not taking anticoagulant therapy |
Thrombo-embolic cerebrovascular event |
||||||||||||
|
Heart failure |
People with heart failure diagnosis |
Not taking angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor–neprilysin inhibitor |
Congestive heart failure or fluid overload |
||||||||||||
|
Cardiovascular disease (a) |
People with cardiovascular disease diagnosis |
Not taking indicated statin |
Ischaemic event |
||||||||||||
|
Cardiovascular disease (b) |
People with cardiovascular disease diagnosis AND No diagnosis of atrial fibrillation |
Not taking indicated antiplatelet medication |
Ischaemic event |
||||||||||||
|
Type 2 diabetes |
People with type 2 diabetes diagnosis ANDPrescribed at least one glucose-lowering medication |
No glycated haemoglobin monitoring test result recorded during past six months |
Diabetes-related complication |
||||||||||||
|
Asthma/congestive obstructive pulmonary disease |
People with previous diagnosis of asthma or congestive obstructive pulmonary disease ANDFrequent use of short-acting beta agonists or muscarinic antagonists (at least two prescriptions in the preceding twelve months) |
No current use of maintenance therapy |
Asthma or congestive obstructive pulmonary disease exacerbation |
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 4 – The ACTMed trial: secondary outcome measures
|
Outcome |
Time point |
||||||||||||||
|
|
|||||||||||||||
|
Change from pre-intervention to intervention period in the proportion of people at risk with a serious medication-related problem (practice-level analysis) |
One month after final practice implements the intervention |
||||||||||||||
|
Change after end of intervention in proportion of people with a serious medication-related problem (persistence of effect) |
Five months after final practice implements the intervention |
||||||||||||||
|
Change from pre-intervention to intervention period in the rate of potentially preventable medication-related hospital admissions relevant to trial clinical indicators (data linkage of Queensland Health hospitalisations data) |
Twelve months after final practice implements the intervention |
||||||||||||||
|
Change from pre-intervention to intervention period in the rate of deaths attributed to medication-related problem (data linkage of Queensland state deaths registry) |
Twelve months after final practice implements the intervention |
||||||||||||||
|
Numbers and types of general practitioner and pharmacist actions for resolving medication-related problems, as entered in Future Health Today as part of the intervention (composite outcome) |
One month after final practice implements the intervention |
||||||||||||||
|
Patient experience of the ACTMed intervention (Consultation And Relational Empathy [CARE] patient feedback measure25 and questions about coordination of care) |
Offered to all patients recalled to the practice for review after the intervention |
||||||||||||||
|
Change between baseline and six months after intervention commencement in health practitioner job satisfaction |
Baseline and one month after final practice implements the intervention |
||||||||||||||
|
Rates of incorrect identification of people as being at risk, by clinical indicator, identified by trial pharmacists during triage |
One month after final practice implements the intervention |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
- Jean Spinks1
- Richard Violette1,2
- Douglas IR Boyle3
- Dennis Petrie4
- Laura Fanning4,5
- Kerry K Hall2
- Fiona Kelly2
- Amanda J Wheeler6,7
- Robert S Ware6
- Joshua Byrnes8
- Esa Chen4
- Andrew Donald9
- Nicolette Ellis10
- Megan DelDot1
- Lisa Nissen1
- Jean Spinks1
- Richard Violette1,2
- Douglas IR Boyle3
- Dennis Petrie4
- Laura Fanning4,5
- Kerry K Hall2
- Fiona Kelly2
- Amanda J Wheeler6,7
- Robert S Ware6
- Joshua Byrnes8
- Esa Chen4
- Andrew Donald9
- Nicolette Ellis10
- Megan DelDot1
- Lisa Nissen1
- 1 Centre for the Business and Economics of Health, the University of Queensland, Brisbane, QLD
- 2 Griffith University, Gold Coast, QLD
- 3 HaBIC Research Information Technology Unit, the University of Melbourne, Melbourne, VIC
- 4 Centre for Health Economics, Monash University, Melbourne, VIC
- 5 Box Hill Hospital, Melbourne, VIC
- 6 Menzies Health Institute of Queensland, Griffith University, Gold Coast, QLD
- 7 The University of Auckland, Auckland, New Zealand
- 8 Centre for Applied Health Economics, Griffith University, Brisbane, QLD
- 9 The University of Melbourne, Melbourne, VIC
- 10 South Brisbane Primary Health Network, Brisbane, QLD
- 1 Centre for the Business and Economics of Health, the University of Queensland, Brisbane, QLD
- 2 Griffith University, Gold Coast, QLD
- 3 HaBIC Research Information Technology Unit, the University of Melbourne, Melbourne, VIC
- 4 Centre for Health Economics, Monash University, Melbourne, VIC
- 5 Box Hill Hospital, Melbourne, VIC
- 6 Menzies Health Institute of Queensland, Griffith University, Gold Coast, QLD
- 7 The University of Auckland, Auckland, New Zealand
- 8 Centre for Applied Health Economics, Griffith University, Brisbane, QLD
- 9 The University of Melbourne, Melbourne, VIC
- 10 South Brisbane Primary Health Network, Brisbane, QLD
Open access:
Open access publishing facilitated by The University of Queensland, as part of the Wiley – The University of Queensland agreement via the Council of Australian University Librarians.
The ACTMed project has been developed by researchers at the University of Queensland, the University of Melbourne, Monash University, and Griffith University, in partnership with Brisbane South Primary Health Network, the Pharmaceutical Society of Australia, the Australian Digital Health Agency, the National Aboriginal Community Controlled Health Organization, MedAdvisor, and the Pharmacy Guild of Australia. We acknowledge the generous contribution of the ACTMed steering committee to project oversight and governance, that of the ACTMed consumer advisory group, particularly Elizabeth Miller and Peter Button, to valuable revisions of the manuscript, and the contributions of non‐partner organisations, including Pharmaceutical Defence Limited. We used de‐identified patient data from the Patron primary care data repository (extracted from consenting general practices) operated by the Melbourne Medical School, the University of Melbourne (www.gp.unimelb.edu.au/datafordecisions).
No relevant disclosures.
- 1. Australian Commission on Safety and Quality in Health Care. National safety and quality health service standards. Sept 2012. www.safetyandquality.gov.au/sites/default/files/migrated/NSQHS‐Standards‐Sept‐2012.pdf (viewed Mar 2022).
- 2. Roughead EE, Semple SJ. Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008. Aust New Zealand Health Policy 2009; 6: 18.
- 3. Roughhead L, Semple S, Rosenfeld E. Literature review: medication safety in Australia. Australian Commission on Safety and Quality in Health Care; Aug 2013. www.safetyandquality.gov.au/sites/default/files/migrated/Literature‐Review‐Medication‐Safety‐in‐Australia‐2013.pdf (viewed Mar 2022).
- 4. Spinks J, Birch S, Wheeler AJ, et al. Provision of home medicines reviews in Australia: linking population need with service provision and available pharmacist workforce. Aust Health Rev 2020; 44: 973‐982.
- 5. Donaldson LJ, Kelley ET, Dhingra‐Kumar N, et al. Medication without harm: WHO's third global patient safety challenge. Lancet 2017; 389: 1680‐1681.
- 6. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15‐19.
- 7. Lim R, Ellett LMK, Semple S, Roughead EE. The extent of medication‐related hospital admissions in Australia: a review from 1988 to 2021. Drug Saf 2022; 45: 249‐257.
- 8. Pharmaceutical Society of Australia. Medicine safety: take care. Jan 2019. www.psa.org.au/wp‐content/uploads/2019/01/PSA‐Medicine‐Safety‐Report.pdf (viewed Mar 2022).
- 9. Pharmacy Programs Adminstrator. Medication medicine programs. 2022. https://www.ppaonline.com.au/programs/medication‐management‐programs (viewed Mar 2022).
- 10. Holland R, Desborough J, Goodyer L, et al. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol 2008; 65: 303‐316.
- 11. Huiskes VJB, Burger DM, van den Ende CHM, van den Bemt BJF. Effectiveness of medication review: a systematic review and meta‐analysis of randomized controlled trials. BMC Fam Pract 2017; 18: 5.
- 12. Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost‐effectiveness analysis. Lancet 2012; 379: 1310‐1319.
- 13. Dreischulte T, Donnan P, Grant A, et al. Safer prescribing: a trial of education, informatics, and financial incentives. N Engl J Med 2016; 374: 1053‐1064.
- 14. Dreischulte T, Grant A, Donnan P, et al. A cluster randomised stepped wedge trial to evaluate the effectiveness of a multifaceted information technology‐based intervention in reducing high‐risk prescribing of non‐steroidal anti‐inflammatory drugs and antiplatelets in primary medical care: the DQIP study protocol. Implement Sci 2012; 7: 24.
- 15. Guthrie B, Kavanagh K, Robertson C, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ 2016; 354: i4079.
- 16. Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015; 350: h391.
- 17. Australian Commission on Safety and Quality in Health Care. Medication reconciliation. Undated. www.safetyandquality.gov.au/our‐work/medication‐safety/medication‐reconciliation (viewed May 2023).
- 18. Brisbane South Primary Health Network. Quality improvement. Quality improvement. Undated. www.bsphn.org.au/support/for‐your‐practice/quality‐improvement/#tools‐and‐toolkits (viewed Mar 2023).
- 19. Kalisch LM, Caughey GE, Barratt JD, et al. Prevalence of preventable medication‐related hospitalizations in Australia: an opportunity to reduce harm. Int J Qual Health Care 2012; 24: 239‐249.
- 20. Spinks JM, Kalisch Ellett LMK, Spurling G, et al. Adaptation of potentially preventable medication‐related hospitalisation indicators for Indigenous populations in Australia using a modified Delphi technique. BMJ Open 2019; 9: e031369.
- 21. Wheeler AJ, Spinks J, Kelly F, et al. Protocol for a feasibility study of an Indigenous Medication Review Service (IMeRSe) in Australia. BMJ Open 2018; 8: e026462.
- 22. Melbourne Medical School. Data for Decisions and the Patron program of research. Undated. www.gp.unimelb.edu.au/datafordecisions (viewed Mar 2022).
- 23. Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972; 62: 337‐342.
- 24. National Heart Foundation of Australia. Clinical fact sheet: stroke prevention in non‐valvular atrial fibrillation using CHA2DS2‐VA score. 2019. www.heartfoundation.org.au/getmedia/eb9cefa4‐c201‐478b‐a787‐5402beaccc0a/Clinical_Fact_Sheet_‐_Stroke_AF.pdf (viewed Feb 2023).
- 25. Mercer SW, Maxwell M, Heaney D, Watt GCM. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy‐based consultation process measure. Fam Pract 2004; 21: 699‐705.
- 26. PRIMIS Team (University of Nottingham). PINCER national rollout. Progress report to NHS England and the AHSN Network. Extended executive summary. July 2020. www.nottingham.ac.uk/primis/documents/pincer/pincer‐progress‐report‐ext‐exec‐summary‐july‐2020.pdf (viewed Feb 2023).
- 27. Hemming K, Girling A. A menu‐driven facility for power and detectable‐difference calculations in stepped‐wedge cluster‐randomized trials. Stata J 2014; 14: 363‐380.
- 28. Royal Australian College of General Practitioners. Standards for general practices. Fifth edition. Melbourne: RACGP, 2020. www.racgp.org.au/FSDEDEV/media/documents/Advocacy/Standards‐for‐general‐practice‐5th‐edition.pdf (viewed Mar 2022)
- 29. Independent Health and Aged Care Pricing Authority. ICD‐10‐AM/ACHI/ACS. Twelfth edition. 15 June 2023. www.ihacpa.gov.au/resources/icd‐10‐amachiacs‐twelfth‐edition (viewed Feb 2023).
- 30. National Health and Medical Research Council; Australian Research Council; Universities Australia. National Statement on Ethical Conduct in Human Research. 2007, updated 2018. www.nhmrc.gov.au/file/9131/download?token=4Qw7LMvh (viewed Feb 2023).
- 31. Melbourne Medical School. Governance and ethics: data governance committee. In: Data for Decisions and the Patron program of research. 15 Aug 2019. https://medicine.unimelb.edu.au/school‐structure/general‐practice‐and‐primary‐care/research/data‐for‐decisions/about‐us/governance‐and‐ethics (viewed Feb 2023).
- 1. Australian Commission on Safety and Quality in Health Care. National safety and quality health service standards. Sept 2012. www.safetyandquality.gov.au/sites/default/files/migrated/NSQHS‐Standards‐Sept‐2012.pdf (viewed Mar 2022).
- 2. Roughead EE, Semple SJ. Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008. Aust New Zealand Health Policy 2009; 6: 18.
- 3. Roughhead L, Semple S, Rosenfeld E. Literature review: medication safety in Australia. Australian Commission on Safety and Quality in Health Care; Aug 2013. www.safetyandquality.gov.au/sites/default/files/migrated/Literature‐Review‐Medication‐Safety‐in‐Australia‐2013.pdf (viewed Mar 2022).
- 4. Spinks J, Birch S, Wheeler AJ, et al. Provision of home medicines reviews in Australia: linking population need with service provision and available pharmacist workforce. Aust Health Rev 2020; 44: 973‐982.
- 5. Donaldson LJ, Kelley ET, Dhingra‐Kumar N, et al. Medication without harm: WHO's third global patient safety challenge. Lancet 2017; 389: 1680‐1681.
- 6. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15‐19.
- 7. Lim R, Ellett LMK, Semple S, Roughead EE. The extent of medication‐related hospital admissions in Australia: a review from 1988 to 2021. Drug Saf 2022; 45: 249‐257.
- 8. Pharmaceutical Society of Australia. Medicine safety: take care. Jan 2019. www.psa.org.au/wp‐content/uploads/2019/01/PSA‐Medicine‐Safety‐Report.pdf (viewed Mar 2022).
- 9. Pharmacy Programs Adminstrator. Medication medicine programs. 2022. https://www.ppaonline.com.au/programs/medication‐management‐programs (viewed Mar 2022).
- 10. Holland R, Desborough J, Goodyer L, et al. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol 2008; 65: 303‐316.
- 11. Huiskes VJB, Burger DM, van den Ende CHM, van den Bemt BJF. Effectiveness of medication review: a systematic review and meta‐analysis of randomized controlled trials. BMC Fam Pract 2017; 18: 5.
- 12. Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost‐effectiveness analysis. Lancet 2012; 379: 1310‐1319.
- 13. Dreischulte T, Donnan P, Grant A, et al. Safer prescribing: a trial of education, informatics, and financial incentives. N Engl J Med 2016; 374: 1053‐1064.
- 14. Dreischulte T, Grant A, Donnan P, et al. A cluster randomised stepped wedge trial to evaluate the effectiveness of a multifaceted information technology‐based intervention in reducing high‐risk prescribing of non‐steroidal anti‐inflammatory drugs and antiplatelets in primary medical care: the DQIP study protocol. Implement Sci 2012; 7: 24.
- 15. Guthrie B, Kavanagh K, Robertson C, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ 2016; 354: i4079.
- 16. Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015; 350: h391.
- 17. Australian Commission on Safety and Quality in Health Care. Medication reconciliation. Undated. www.safetyandquality.gov.au/our‐work/medication‐safety/medication‐reconciliation (viewed May 2023).
- 18. Brisbane South Primary Health Network. Quality improvement. Quality improvement. Undated. www.bsphn.org.au/support/for‐your‐practice/quality‐improvement/#tools‐and‐toolkits (viewed Mar 2023).
- 19. Kalisch LM, Caughey GE, Barratt JD, et al. Prevalence of preventable medication‐related hospitalizations in Australia: an opportunity to reduce harm. Int J Qual Health Care 2012; 24: 239‐249.
- 20. Spinks JM, Kalisch Ellett LMK, Spurling G, et al. Adaptation of potentially preventable medication‐related hospitalisation indicators for Indigenous populations in Australia using a modified Delphi technique. BMJ Open 2019; 9: e031369.
- 21. Wheeler AJ, Spinks J, Kelly F, et al. Protocol for a feasibility study of an Indigenous Medication Review Service (IMeRSe) in Australia. BMJ Open 2018; 8: e026462.
- 22. Melbourne Medical School. Data for Decisions and the Patron program of research. Undated. www.gp.unimelb.edu.au/datafordecisions (viewed Mar 2022).
- 23. Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972; 62: 337‐342.
- 24. National Heart Foundation of Australia. Clinical fact sheet: stroke prevention in non‐valvular atrial fibrillation using CHA2DS2‐VA score. 2019. www.heartfoundation.org.au/getmedia/eb9cefa4‐c201‐478b‐a787‐5402beaccc0a/Clinical_Fact_Sheet_‐_Stroke_AF.pdf (viewed Feb 2023).
- 25. Mercer SW, Maxwell M, Heaney D, Watt GCM. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy‐based consultation process measure. Fam Pract 2004; 21: 699‐705.
- 26. PRIMIS Team (University of Nottingham). PINCER national rollout. Progress report to NHS England and the AHSN Network. Extended executive summary. July 2020. www.nottingham.ac.uk/primis/documents/pincer/pincer‐progress‐report‐ext‐exec‐summary‐july‐2020.pdf (viewed Feb 2023).
- 27. Hemming K, Girling A. A menu‐driven facility for power and detectable‐difference calculations in stepped‐wedge cluster‐randomized trials. Stata J 2014; 14: 363‐380.
- 28. Royal Australian College of General Practitioners. Standards for general practices. Fifth edition. Melbourne: RACGP, 2020. www.racgp.org.au/FSDEDEV/media/documents/Advocacy/Standards‐for‐general‐practice‐5th‐edition.pdf (viewed Mar 2022)
- 29. Independent Health and Aged Care Pricing Authority. ICD‐10‐AM/ACHI/ACS. Twelfth edition. 15 June 2023. www.ihacpa.gov.au/resources/icd‐10‐amachiacs‐twelfth‐edition (viewed Feb 2023).
- 30. National Health and Medical Research Council; Australian Research Council; Universities Australia. National Statement on Ethical Conduct in Human Research. 2007, updated 2018. www.nhmrc.gov.au/file/9131/download?token=4Qw7LMvh (viewed Feb 2023).
- 31. Melbourne Medical School. Governance and ethics: data governance committee. In: Data for Decisions and the Patron program of research. 15 Aug 2019. https://medicine.unimelb.edu.au/school‐structure/general‐practice‐and‐primary‐care/research/data‐for‐decisions/about‐us/governance‐and‐ethics (viewed Feb 2023).






Abstract
Background: Medicines are the most frequent health care intervention type; their safe use provides significant benefits, but inappropriate use can cause harm. Systemic primary care approaches can manage serious medication‐related problems in a timely manner.
Objectives: ACTMed (ACTivating primary care for MEDicine safety) uses information technology and financial incentives to encourage pharmacists to work more closely with general practitioners to reduce the risk of harm, improve patients’ experience of care, streamline workflows, and increase the efficiency of medical care.
Methods and analysis: The stepped wedge cluster randomised trial in 42 Queensland primary care practices will assess the effectiveness of the ACTMed intervention. The primary outcome will be the proportion of people at risk of serious medication‐related problems — patients with atrial fibrillation, heart failure, cardiovascular disease, type 2 diabetes, or asthma or congestive obstructive pulmonary disease — who experience such problems. We will also estimate the cost per averted serious medication‐related problem and the cost per averted potentially preventable medication‐related hospitalisation.
Ethics approval: The University of Queensland Human Research Ethics Committee approved the pilot (2021/HE002189) and trial phases of the ACTMed study (2022/HE002136). Access to Patron data was granted by the Patron Data Governance Committee (PAT052ACTMed). Access to linked hospitalisations and deaths data are subject toPublic Health Act approval (pending).
Dissemination of findings: A comprehensive dissemination plan will be co‐developed by the researchers, the ACTMed steering committee and consumer advisory group, project partners, and trial site representatives. Aboriginal and Torres Strait Islander communities will be supported in leading community‐level dissemination.
Trial registration: Australian New Zealand Clinical Trials Registry (pilot: ACTRN12622000595718; 21 April 2022; full trial: ACTRN12622000574741; 14 April 2022).