White matter hyperintensities (WMH) of presumed vascular origin are areas of increased signal in the cerebral white matter, best seen on T2 weighted and fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) sequences.1 They are commonly present in about 50% of individuals in their fifth decade2 and in up to 95% of people by age 90 years.3 These lesions were considered of little clinical importance, but current evidence suggests that they portend poor brain and cardiovascular health, including increased risk of stroke, cognitive decline and dementia, gait impairments, later life depression, and death.4,5,6 Little clinical guidance exists regarding the best management of incidentally found WMH, leaving many health practitioners no option but referral to the neurology outpatient clinic. To address this need, this consensus statement was formed as a guide to investigation and management for general medical practitioners.
Pathophysiology of WMH
The pathophysiology of WMH is heterogenous and incompletely understood. Radiologically, these white matter lesions indicate increased water content. Potential aetiologies include demyelination, inflammation, trauma, neoplasm, degeneration, infarction and ischaemia. WMH of presumed vascular origin are thought to be due to chronic ischaemia via age‐related degeneration of perforating arterioles, lipohyalinosis, and blood–brain barrier dysfunction.7
Radiological appearance
WMH are areas of MRI T2 hyperintense signal in the cerebral white matter. They are generally bilateral and symmetrical,1 and have many names, including leukoaraiosis and white matter lesions.1 WMH may be visible on computed tomography imaging of the brain as low density (hypoattenuation) white matter lesions.8 Radiologists have expertise in the differentiation of WMH of presumed vascular origin from other aetiologies. Their report will benefit from relevant clinical information.
Medical practitioners should be guided by the expert radiology report if a specific diagnosis is raised. However, in patients aged 50 years and older, most white matter lesions are not assigned a specific syndrome.1
Although WMH rating scales are rarely used in clinical practice, for research purposes, WMH severity is often graded with the Fazekas score.9 Higher Fazekas scores and more confluent WMH have been associated with worse cognitive, clinical and functional outcomes.4 Periventricular WMH, confluent with the lateral ventricles or within 10 mm of the lateral ventricle border,10 have been associated with poorer cognitive performance than deep WMH, although this is not consistent across all studies (Box 1).11
Risk factors
Recognised risk factors for WMH are cardiovascular risk factors, especially age and hypertension.12 Modifiable risk factors also include smoking13 and obstructive sleep apnoea.14,15 The discovery of WMH presents an opportunity for investigation and management of such factors, potentially improving both brain and general health.
We note that WMH are found commonly in migraineurs,16 but without cardiovascular risk factors they do not confer the same risk as in patients with risk factors.17 People with epilepsy also have greater WMH burden and this is posited as a contributor to their increased risk of stroke.18 Several monogenic cerebral small‐vessel diseases are associated with characteristic WMH patterns, but discussion and recommendations for these conditions are not included in this statement.19
Methods
The aim of this consensus statement is to help clinicians to implement appropriate investigation and management for adults with incidental WMH and improve detection of cardiovascular risk factors and avoid potentially harmful therapies. The need for neurology‐driven guidance on the management of incidentally found WMH was identified at the 2021 Australasian Stroke Academy Conference. A writing group consisting of a specialist cognitive neurologist, general neurologist and basic physician trainee was formed. The writing group agreed that the population of interest would be adults (≥ 18 years old) with incidental WMH, with a focus on screening, primary and secondary prevention of stroke and cardiovascular disease via pharmacological and lifestyle interventions, and primary and secondary prevention of dementia risk factors. Comparators were either best medical care or placebo, and the outcomes would be the reduction of risk of stroke, cognitive decline, or dementia.
Given the anticipated paucity of primary studies on this topic, this consensus statement was developed through a literature review and expert opinion. The Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument20 was used to guide development, and the quality of evidence for key recommendations was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework (Box 2).21
Search strategy
One author (TO) conducted a search of PubMed for all time periods until 20 November 2022. The search terms used were “white matter hyperintensities”, “leukoaraiosis”, “white matter lesions” and “leukoencephalopathy”, plus each relevant risk factor or potential intervention. Priority was given to systematic reviews and meta‐analyses of either randomised controlled trials (RCTs) or observational studies and to individual observational studies or RCTs if systematic reviews and meta‐analyses were not found. Publications were limited to English.
Expert recommendations
A modified Delphi approach22 was adopted and an expert panel assembled to inform recommendations in this consensus statement. The panel comprised 14 Australian and New Zealand neurologists with at least three years consultant experience in vascular and/or cognitive neurology. Eight questions regarding investigation and management of incidentally found WMH were answered with either yes or no. A summary of the expert panel's opinion is presented in Box 3 and the questions are provided in the Supporting Information.
External review
To ensure applicability and practicality, we consulted clinicians in relevant specialties. For example, neuroradiologists contributed to writing and editing relevant sections, and expert general physicians, geriatricians, psychiatrists, general practitioners, and general and neurologists also reviewed this consensus statement before publication. Comments from these clinicians were incorporated into the final manuscript. The Australasian Stroke Academy endorses this consensus statement.
Recommendations
Antihypertensive therapy
Antihypertensive therapy is an effective intervention to reduce the risk of cardiovascular events and is an important risk factor for presence and progression of WMH.13 A meta‐analysis found that antihypertensive medication had a modest effect on WMH progression (standardised mean difference [SMD], −0.19; 95% confidence interval [CI], −0.32 to −0.06).23 One RCT included in this meta‐analysis suggested that WMH volume reduction did not protect against or reverse cognitive decline.24 There is a small literature regarding stroke survivors wherein intensive antihypertensive treatment was associated with Fazekas score‐graded WMH reduction and reduced stroke recurrence, especially in patients with the greatest blood pressure reduction.25
Another meta‐analysis reported a similar effect of antihypertensive therapy on WMH (SMD, −0.22; 95%CI, −0.36 to −0.07).26 To investigate optimal blood pressure targets, the meta‐analysis authors performed a subgroup analysis, stratifying by blood pressure at follow‐up, and found the effect of antihypertensive medication on WMH volume remained significant only if a systolic blood pressure of 110–129 mmHg was achieved.26 We note that the Canadian Consensus Conference on Diagnosis and Treatment of Dementia recommends targeting a systolic blood pressure of less than 120 mmHg in middle‐aged individuals with vascular risk factors, positing this may confer modest protection against developing mild cognitive impairment.27 The SPRINT‐MIND trial, an RCT investigating systolic blood pressure targets of 140 mmHg versus 120 mmHg on the risk of probable dementia, found no difference in incidence of probable dementia between the two groups,28 but the study may not have been adequately powered to detect a difference.
In summary, the association between hypertension and WMH is the most well studied of all risk factors. Evidence from meta‐analyses supports a beneficial effect of blood pressure reduction on WMH burden. The clinical benefit on cognition of reducing WMH volume is yet to be fully determined (Box 4).
Statins
A systematic review and meta‐analysis of RCTs investigating statin therapy and risk of covert infarcts found that patients randomly assigned to statin treatment had a lower risk of new covert infarcts (relative risk, 0.63; 95% CI, 0.46–0.88; P = 0.006).29 However, heterogenous participants were recruited30,31,32 and different statins were used. Of these studies, only one RCT of older Chinese people with carotid atherosclerotic disease33 using both sartans and statins reported a change in WMH volume in a subgroup analysis. Patients assigned to rosuvastatin 10 mg daily had a significantly lower increase in WMH volume after an average follow‐up time of 61.8 months.30 A secondary outcome from this trial was incidence of mild cognitive impairment, defined by a score of 23 or below or a decline by three points or less on the Mini‐Mental State Examination (MMSE) or a score of 123 or less on the dementia rating scale at any follow‐up visit.33 The telmisartan versus placebo arm showed no difference in incidence of mild cognitive impairment, but the rosuvastatin versus placebo arm revealed a decrease in mild cognitive impairment (hazard ratio, 0.54; 95% CI, 0.36‐0.80).33
Montreal Cognitive Assessment (MoCA) scores increased from an average of 18 to 22 in the rosuvastatin plus nimodipine group and from 17 to 19 in the control group after six months of treatment in another RCT of mild cognitive impairment in patients with cerebral small vessel disease (P < 0.05).34 Although this may support statin treatment in patients with mild cognitive impairment, it cannot be extrapolated to those in whom WMH are purely incidental.
In summary, direct evidence is lacking for statin treatment for incidentally found WMH. The above studies were conducted in populations that were older, had established cardiovascular disease or mild cognitive impairment, and combined statins with antihypertensives, making untangling the effect of statins alone difficult. Therefore, no specific recommendation for statin treatment is made in this consensus statement, other than to follow existing lipid‐lowering guidelines (Box 5).
Anticoagulants and antiplatelet medications
Aspirin
Aspirin is proven therapy for secondary prevention of stroke. The ASPREE study conclusively demonstrated that aspirin was not indicated for primary prevention of stroke owing to a greater risk of adverse effects, including intracerebral haemorrhage.35 There is no evidence for the role of aspirin in the treatment of incidental WMH. Canadian‐based guidelines for the management of vascular cognitive impairment recommend aspirin in patients with stroke and mild cognitive impairment or dementia and WMH, but not for those with no history of stroke.27
In summary, the increased risk of harm from aspirin use for primary prevention has been established in the ASPREE study.35 Although primary treatment of WMH with aspirin has not been systematically examined, international guidelines do not support aspirin use in patients with WMH and mild cognitive impairment or dementia without demonstrated stroke (Box 6).
Anticoagulants
The COMPASS study randomly assigned patients with stable coronary or peripheral artery disease to aspirin 100 mg daily, rivaroxaban 5 mg twice daily or aspirin 100 mg daily plus rivaroxaban 2.5 mg twice daily, and demonstrated a reduced incidence of strokes in the rivaroxaban plus aspirin group.36 No difference in the rate of WMH accrual was observed between patients receiving aspirin plus rivaroxaban versus rivaroxaban alone versus aspirin alone in an associated substudy.37 However, due to early termination, the study was not powered to exclude a clinically important difference and allow conclusions about the effect of rivaroxaban on WMH accrual (Box 7).
Diet and exercise interventions
Metabolic syndrome — defined as central adiposity, triglycerides greater than 1.7 mmol/L, high‐density lipoprotein cholesterol less than 1.0 mmol/L in men and less than 1.3 mmol/L in women, systolic blood pressure greater than 130 mmHg and/or diastolic blood pressure greater than 85 mmHg, and fasting blood glucose greater than 5.5 mmol/L38 — is a known risk factor for stroke and cardiovascular disease.39 Cross‐sectional studies have supported an association between metabolic syndrome and WMH progression39,40 but this is less clear in prospective studies. An analysis of participants of the Mayo Clinic Study of Aging who had completed at least two MRI brain scans found that age, hypertension (especially in women), and impaired fasting glucose (especially in men) were all independently associated with WMH progression, but metabolic syndrome was not a significant independent risk factor.12 However, when individuals with type 2 diabetes were randomly assigned to an intensive diet and exercise intervention or support and education arms, the intensive intervention group had significantly smaller WMH volumes at follow‐up ten to 12 years later.41 There was no evidence that this protected against cognitive decline. However, in a three‐year observational study of cognitively normal adults with WMH, those with a greater level of physical activity were less likely to progress to cognitive decline or dementia.42 There is some evidence that resistance training for cognitively normal women aged 65–75 years was associated with lower WMH volumes and better maintenance of gait speed, but no difference in executive function, compared with those in a balance and tone exercise arm of the study.43 Prevention of stroke was not an outcome assessed in these studies but there is good evidence for lifestyle intervention in reducing risk of stroke and is reflected in guidelines.44,45,46
In summary, prospective studies have demonstrated that a reduction in WMH accrual is possible with the treatment of metabolic syndrome;41,43 however, it is yet to be determined whether this benefits cognition. We recommend following existing guidelines for diet and exercise45,46 for optimal cardiovascular and brain health (Box 8).
Smoking cessation
There is a strong association between smoking and WMH.12,13 In addition to being a vascular risk factor, there is evidence that smoking directly interferes with white matter integrity.47 A prospective cohort study of almost 1000 individuals who completed MRI brain scans ten years apart found a dose–response relationship between smoking and WMH.48 There are no specific recommendations for smoking cessation in subclinical cerebrovascular disease.49 The specific clinical benefit of reducing smoking for WMH is not established, but given the benefits for brain health, smoking cessation is recommended in people with incidental WMH.
In summary, smoking has been shown to be associated with increased WMH; however, the effects on cognition and stroke risk were not examined.48 Nevertheless, smoking cessation is a routine general preventive health measure (Box 8).
Obstructive sleep apnoea
The association between obstructive sleep apnoea (OSA) and adverse cardiovascular events including stroke is strong,50 but the association with WMH is less clear. Cross‐sectional studies have revealed correlations between moderate to severe OSA51 and OSA in older patients52 with WMH prevalence. A meta‐analysis of nine cross‐sectional and cohort studies comparing the incidence of WMH in individuals with and without OSA found an odds ratio for WMH incidence of 2.31 (95% CI, 1.46–3.66) compared with those without OSA.14 Another systematic review and meta‐analysis found that moderate to severe OSA, but not mild OSA, was significantly associated with WMH incidence.53 There is variable evidence that adherence to continuous positive airway pressure for treatment of OSA may reduce risk of stroke.54
In summary, evidence for an association between OSA and WMH accrual is provided by two systematic reviews and meta‐analyses,14,52 but the effect of OSA treatment on WMH reduction or prevention of progression is not yet determined. The evidence supports a dose–response relationship, so determining the effect of preventing progression of OSA on WMH accrual would be beneficial (Box 9).
When to refer
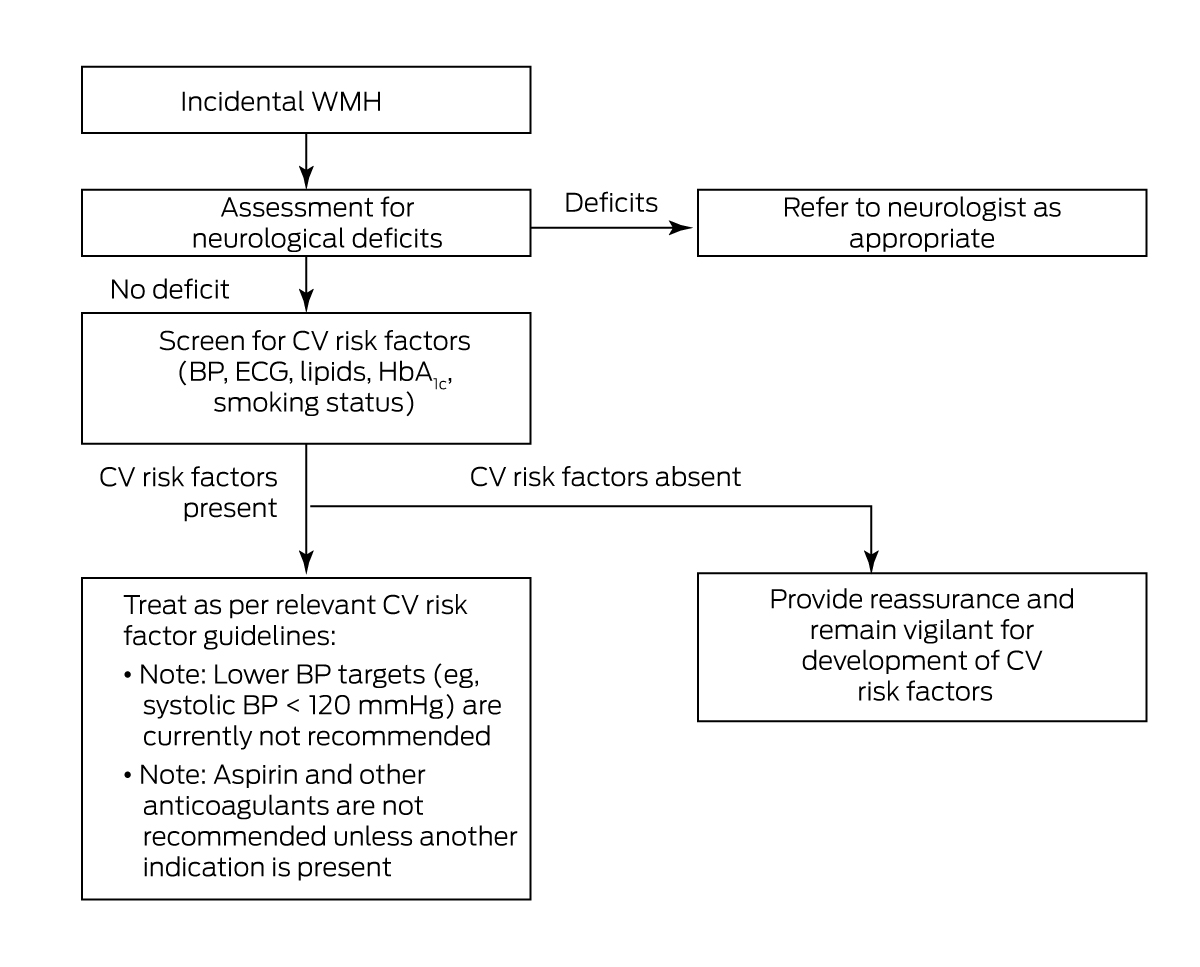
Patients with incidentally found WMH are commonly referred to outpatient neurology clinics. This may be driven by concern regarding neurological symptoms, or the radiological differential diagnosis of WMH. In general, a neurology referral is warranted when symptoms and signs are rapidly progressive or unexplained, or when radiological features are atypical for WMH of presumed vascular origin as outlined above (Box 10).
Conclusions
Incidentally found WMH on neuroimaging may be considered an opportunity to screen for and address cardiovascular risk factors to prevent progression to cognitive or other neurological impairment. With the help of this consensus statement, such management can be implemented by general practitioners and non‐neurologist physicians. Neurology referral may only be necessary for rapidly progressive or atypical symptoms and atypical radiological findings.
Box 1 – Magnetic resonance imaging scans representing Fazekas grades*
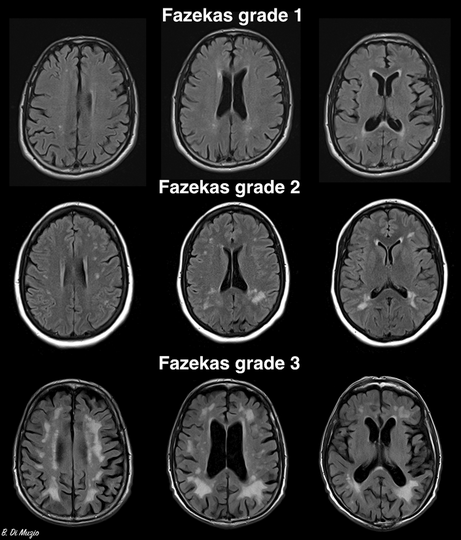
* The Fazekas grading system divides white matter hyperintensities into deep and periventricular lesions, and grades each region separately: deep: 0 = absent, 1 = punctate foci, 2 = early confluence, 3 = large areas of confluence; periventricular: 0 = absent, 1 = thin lining or “caps” on lateral ventricles, 2 = smooth halo around lateral ventricles, 3 = irregular periventricular signal extending to the deep white matter.9The top row of the figure demonstrates punctate foci and caps on the lateral ventricles; the middle row demonstrates early confluence and smooth halo around lateral ventricles; and the bottom row demonstrates large areas of confluence in the deep white matter and irregular periventricular signal extending to the deep white matter.Source: Image reproduced from Di Muzio B. Fazekas scale for white matter lesions. Case study. Radiopaedia.org doi: . https://creativecommons.org/licenses/by‐nc‐sa/3.0/legalcode (viewed July 2023).
Box 2 – Summary of recommendations, quality of evidence, and expert opinion for interventions for incidental white matter hyperintensities (WMH)
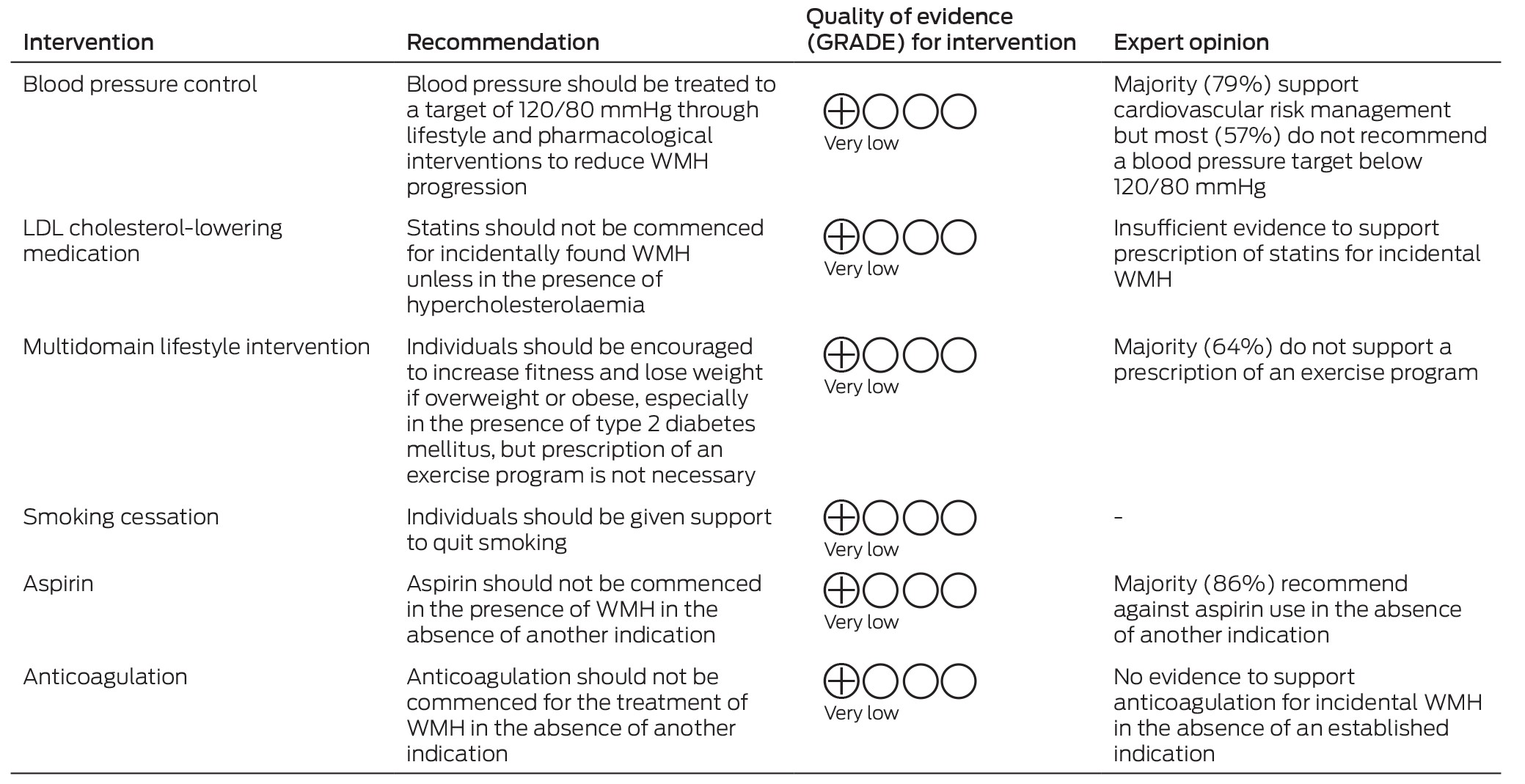
GRADE = Grading of Recommendations, Assessment, Development and Evaluation; LDL = low‐density lipoprotein.
Box 3 – Summary of recommendations and expert opinion for investigations for incidental white matter hyperintensities (WMH)
|
Recommendation |
Expert opinion |
||||||||||||||
|
|
|||||||||||||||
|
Screening should include investigation of lipids, HbA1c level, blood pressure, smoking status, and ECG |
Majority (93%) support cardiovascular risk screening |
||||||||||||||
|
Screening for OSA |
Majority (64%) do not support screening for OSA |
||||||||||||||
|
24‐Hour ambulatory blood pressure monitoring |
Majority (71%) do not support 24‐hour ambulatory blood pressure monitoring |
||||||||||||||
|
Screening for cognitive impairment |
Majority (64%) do not support formal screening for cognitive impairment |
||||||||||||||
|
|
|||||||||||||||
|
ECG = electrocardiogram; HbA1c = glycated haemoglobin; OSA = obstructive sleep apnoea. |
|||||||||||||||
Box 4 – Summary of evidence for antihypertensive therapy on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Antihypertensives |
|||||||||||||||
|
Effect on WMH: Modest effect for reducing progression |
|||||||||||||||
|
Effect on cognition: Possible modest effect if systolic blood pressure is < 120 mmHg |
|||||||||||||||
|
Effect on stroke risk: Possible reduction in stroke risk |
|||||||||||||||
|
Recommendations:
|
|||||||||||||||
|
Advice to patients: WMH may represent damage to the brain from uncontrolled blood pressure. Lowering blood pressure will lead to a reduced risk of progression of WMH and reduce the risk of strokes and heart attacks |
|||||||||||||||
|
|
|||||||||||||||
|
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Box 5 – Summary of evidence for statin therapy on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Statins |
|||||||||||||||
|
Effect on WMH: May reduce accumulation |
|||||||||||||||
|
Effect on cognition: Not adequately studied in patients with incidental WMH |
|||||||||||||||
|
Effect on stroke risk: May reduce risk if strong cardiovascular disease risk factors are present |
|||||||||||||||
|
Recommendation: Statins should not be commenced for incidentally found WMH
|
|||||||||||||||
|
Advice to patients: Although high cholesterol may be contributing to WMH burden, there is not enough evidence to support commencing medications without another indication. Healthy lifestyle changes are still advisable |
|||||||||||||||
|
|
|||||||||||||||
|
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Box 6 – Summary of evidence for antiplatelet therapy (aspirin) on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Aspirin |
|||||||||||||||
|
Effect on WMH: Unknown |
|||||||||||||||
|
Effect on cognition: None |
|||||||||||||||
|
Effect on stroke risk: Decreased only when used for secondary prevention |
|||||||||||||||
|
Recommendation: Aspirin should not be commenced in the presence of WMH in the absence of another indication
|
|||||||||||||||
|
Advice to patients: The risk of bleeding due to aspirin will outweigh the benefit of this medication if used purely for treatment of incidental WMH |
|||||||||||||||
|
|
|||||||||||||||
|
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Box 7 – Summary of evidence for anticoagulation therapy on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Low dose anticoagulation |
|||||||||||||||
|
Effect on WMH: Unknown |
|||||||||||||||
|
Effect on cognition: Unknown |
|||||||||||||||
|
Effect on stroke risk: Evidence of reduced risk of stroke in people with stable peripheral or coronary artery disease for combination of low dose rivaroxaban and aspirin compared with aspirin alone, but rivaroxaban monotherapy did not reduce ischaemic stroke and increased risk of haemorrhagic stroke36 |
|||||||||||||||
|
Recommendation: Anticoagulation should not be commenced for the treatment of WMH in the absence of another indication
|
|||||||||||||||
|
Advice to patients: The risk of bleeding due to anticoagulation will outweigh the benefit of this medication if used purely for treatment of incidental WMH |
|||||||||||||||
|
|
|||||||||||||||
|
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Box 8 – Summary of evidence for lifestyle intervention on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Diet, exercise, and smoking cessation |
|||||||||||||||
|
Effect on WMH: May reduce progression |
|||||||||||||||
|
Effect on cognition: Further studies required |
|||||||||||||||
|
Effect on stroke risk: Reduces risk |
|||||||||||||||
|
Recommendations:
|
|||||||||||||||
|
Advice to patients: Achieving the recommended level of exercise, eating a healthy diet and quitting smoking (if applicable) may reduce WMH progression and will protect against cardiovascular disease |
|||||||||||||||
|
|
|||||||||||||||
|
ECG = electrocardiogram; HbA1c = glycated haemoglobin; GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Box 9 – Summary of evidence for obstructive sleep apnoea (OSA) intervention on white matter hyperintensities (WMH) accumulation and neurological disease risk
|
|
|||||||||||||||
|
Intervention: Treatment of OSA |
|||||||||||||||
|
Effect on WMH: Unknown; possible benefit if moderate or severe OSA |
|||||||||||||||
|
Effect on cognition: Unknown |
|||||||||||||||
|
Effect on stroke risk: May reduce risk if good adherence to continuous positive airway pressure |
|||||||||||||||
|
Recommendations: Patients with WMH may not require specific screening for OSA
|
|||||||||||||||
|
Advice to patients: OSA may be contributing to the presence of WMH. An additional benefit of treating OSA may be reduction in progression of WMH |
|||||||||||||||
|
|
|||||||||||||||
|
GRADE = Grading of Recommendations, Assessment, Development and Evaluation. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Thomas P Ottavi1
- Elizabeth Pepper1
- Grant Bateman1
- Mark Fiorentino1
- Amy Brodtmann2
- 1 John Hunter Hospital, Newcastle, NSW
- 2 Monash University, Melbourne, VIC
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
We thank and acknowledge Darshan Ghia, Bernard Yan, Mark Parsons, Andrew Wong, Neil Spratt, Ben Clissold, Anna Holwell, Henry Ma, Claire Muller, Kenneth Butcher, Candice Delcourt, Teddy Wu, and Anna Ranta for their contributions as the expert panel and their review of the manuscript. We also thank and acknowledge Andrew Lee, Rebecca Moore, Gregory Carter, and Kichu Nair for reviewing the manuscript.
No relevant disclosures.
- 1. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822‐838.
- 2. Wen W, Sachdev PS, Li JJ, et al. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp 2009; 30: 1155‐1167.
- 3. de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9‐14.
- 4. Debette S, Schilling S, Duperron MG, et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta‐analysis. JAMA Neurol 2019; 76: 81‐94.
- 5. Hu HY, Ou YN, Shen XN, et al. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta‐analysis of 36 prospective studies. Neurosci Biobehav Rev 2021; 120: 16‐27.
- 6. Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 2008; 79: 619‐624.
- 7. Lin J, Wang D, Lan L, et al. Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res Int 2017; 2017: 9372050.
- 8. Ferguson KJ, Cvoro V, MacLullich AMJ, et al. Visual rating scales of white matter hyperintensities and atrophy: comparison of computed tomography and magnetic resonance imaging. J Stroke Cerebrovasc Dis 2018; 27: 1815‐1821.
- 9. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol 1987; 149: 351‐356.
- 10. Griffanti L, Jenkinson M, Suri S, et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: a study in older adults. Neuroimage 2018; 170: 174‐181.
- 11. Bolandzadeh N, Davis JC, Tam R, et al. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurology 2012; 12: 126.
- 12. Scharf EL, Graff‐Radford J, Przybelski SA, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: the Mayo Clinic Study of Aging. Stroke 2019; 50: 3037‐3044.
- 13. Brown R, Low A, Markus HS. Rate of, and risk factors for, white matter hyperintensity growth: a systematic review and meta‐analysis with implications for clinical trial design. J Neurol Neurosurg Psychiatry 2021; 92: 1271‐1277.
- 14. Chokesuwattanaskul A, Lertjitbanjong P, Thongprayoon C, et al. Impact of obstructive sleep apnea on silent cerebral small vessel disease: a systematic review and meta‐analysis. Sleep Med 2020; 68: 80‐88.
- 15. Huang Y, Yang C, Yuan R, et al. Association of obstructive sleep apnea and cerebral small vessel disease: a systematic review and meta‐analysis. Sleep 2020; 43: zsz264.
- 16. Bashir A, Lipton RB, Ashina S, et al. Migraine and structural changes in the brain: a systematic review and meta‐analysis. Neurology 2013; 81: 1260‐1268.
- 17. Dobrynina LA, Suslina AD, Gubanova MV, et al. White matter hyperintensity in different migraine subtypes. Sci Rep 2021; 11: 10881.
- 18. Mao YT, Goh E, Churilov L, et al. White matter hyperintensities on brain magnetic resonance imaging in people with epilepsy: a hospital‐based study. CNS Neurosci Ther 2016; 22: 758‐763.
- 19. Mancuso M, Arnold M, Bersano A, et al. Monogenic cerebral small‐vessel diseases: diagnosis and therapy. consensus recommendations of the European Academy of Neurology. Eur J Neurol 2020; 27: 909‐927.
- 20. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ 2010; 182: E472‐E478.
- 21. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490‐1488.
- 22. Trevelyan EG, Robinson PN. Delphi methodology in health research: how to do it? Eur J Integr Med 2015; 7: 423‐428.
- 23. van Middelaar T, Argillander TE, Schreuder FHBM, et al. Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and meta‐analysis. Stroke 2018; 49: 1531‐1533.
- 24. Murray AM, Hsu FC, Williamson JD, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017; 60: 69‐80.
- 25. Wardlaw JM, Chappell FM, Valdés Hernández MDC, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017; 89: 1003‐1010.
- 26. Su C, Wu H, Yang X, et al. The relation between antihypertensive treatment and progression of cerebral small vessel disease: a systematic review and meta‐analysis of randomized controlled trials. Medicine (Baltimore) 2021; 100: e26749‐e26749.
- 27. Smith EE, Barber P, Field TS, et al. Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD) 5: guidelines for management of vascular cognitive impairment. Alzheimers Dementia (N Y) 2020; 6: e12056.
- 28. SPRINT MIND Investigators for the SPRINT Research Group; Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321: 553‐561.
- 29. Katsanos AH, Lioutas VA, Charidimou A, et al. Statin treatment and accrual of covert cerebral ischaemia on neuroimaging: a systematic review and meta‐analysis of randomized trials. Eur J Neurol 2020; 27: 1023‐1027.
- 30. Ji T, Zhao Y, Wang J, et al. Effect of low‐dose statins and apolipoprotein E genotype on cerebral small vessel disease in older hypertensive patients: a subgroup analysis of a randomized clinical trial. J Am Med Dir Assoc 2018; 19: 995‐1002.
- 31. ten Dam VH, van den Heuvel DM, van Buchem MA, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology 2005; 64: 1807‐1809.
- 32. Fu JH, Mok V, Lam W, et al. Effects of statins on progression of subclinical brain infarct. Cerebrovasc Dis 2010; 30: 51‐56.
- 33. Zhang H, Cui Y, Zhao Y, et al. Effects of sartans and low‐dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double‐blind and placebo‐controlled clinical trial. Hypertens Res 2019; 42: 717‐729.
- 34. Zhang J, Liu N, Yang C. Effects of rosuvastatin in combination with nimodipine in patients with mild cognitive impairment caused by cerebral small vessel disease. Panminerva Med 2019; 61: 439‐443.
- 35. McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability‐free survival in the healthy elderly. N Engl J Med 2018; 379: 1499‐1508.
- 36. Sharma M, Hart RG, Connolly SJ, et al. Stroke outcomes in the COMPASS trial. Circulation 2019; 139: 1134‐1145.
- 37. Sharma M, Hart RG, Smith EE, et al. Rivaroxaban for prevention of covert brain infarcts and cognitive decline: the COMPASS MRI substudy. Stroke 2020; 51: 2901‐2909.
- 38. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640‐1645.
- 39. Bokura H, Yamaguchi S, Iijima K, et al. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke 2008; 39: 1607‐1609.
- 40. Park K, Yasuda N, Toyonaga S, et al. Significant association between leukoaraiosis and metabolic syndrome in healthy subjects. Neurology 2007; 69: 974‐978.
- 41. Espeland MA, Erickson K, Neiberg RH, et al. Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care 2016; 39: 764‐771.
- 42. Verdelho A, Madureira S, Ferro JM, et al. Physical activity prevents progression for cognitive impairment and vascular dementia: results from the LADIS (Leukoaraiosis and Disability) study. Stroke 2012; 43: 3331‐3335.
- 43. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12‐month randomized controlled trial. J Am Geriatr Soc 2015; 63: 2052‐2060.
- 44. Kono Y, Yamada S, Yamaguchi J, et al. Secondary prevention of new vascular events with lifestyle intervention in patients with noncardioembolic mild ischemic stroke: a single‐center randomized controlled trial. Cerebrovasc Dis 2013; 36: 88‐97.
- 45. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364‐e467.
- 46. Commonwealth of Australia as represented by the Department of Health and Aged Care. Australian guideline for assessing and managing cardiovascular disease risk, 2023. https://www.cvdcheck.org.au/overview (viewed Aug 2023).
- 47. Gons RAR, van Norden AGW, de Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011; 134: 2116‐2124.
- 48. Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC‐MRI Study. Neurology 2015; 84: 841‐848.
- 49. Wardlaw JM, Debette S, Jokinen H, et al. ESO guideline on covert cerebral small vessel disease. Eur Stroke J 2021; 6: CXI‐CLXII.
- 50. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health 2015; 36: 417‐440.
- 51. Nishibayashi M, Miyamoto M, Miyamoto T, et al. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med 2008; 4: 242‐247.
- 52. Kim H, Yun C‐H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle‐aged and older general population. Sleep 2013; 36: 709‐715.
- 53. Ho BL, Tseng PT, Lai CL, et al. Obstructive sleep apnea and cerebral white matter change: a systematic review and meta‐analysis. J Neurol 2018; 265: 1643‐1653.
- 54. Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. European Journal of Neurology. 2020;27(7):1117‐1136.






Abstract
Introduction: There is a paradigm shift in our understanding of white matter hyperintensities (WMH) found on brain imaging. They were once thought to be a normal phenomenon of ageing and, therefore, warranted no further investigation. However, evidence now suggests these lesions are markers of poor brain and cardiovascular health, portending an increased risk of stroke, cognitive decline, depression and death. Nevertheless, no specific guidelines exist for the management of incidentally found WMH for general medical practitioners and other clinicians ordering brain magnetic resonance imaging scans for diverse clinical indications. Informed by a literature review and expert opinion gleaned from stroke neurologists, medical and imaging specialists, and general practitioners, we present our consensus statement to guide the management of incidentally found WMH in adults.
Main recommendations: When incidental WMH are found on brain imaging:
Changes to management as a result of this consensus statement: A brain health opportunity. We consider the discovery of incidental WMH on brain imaging to represent an opportunity to investigate for common cardiovascular risk factors and to optimise brain health. This can be commenced and monitored by the general practitioner or physician without delay in waiting for an outpatient neurology review.