The World Health Organization aims to eliminate hepatitis C as a health problem by 2030.1 Injecting drug use is the main risk factor for hepatitis C in Australia.2 The introduction of highly effective and well tolerated antiviral therapies led to an early surge in treatment (33 200 people during 2016) that has since ebbed (8100 during 2020).1 It is estimated that 40–45% of Australians who need treatment have received it.1 Explanations for the decline in the number treated include low engagement with the medical system, the falling prevalence of hepatitis C, and ambivalence about treatment related to lack of trust in medical staff, fear of side effects, and competing social and health priorities, especially for people with asymptomatic hepatitis C virus (HCV) infections.3
The Australian Treatment Outcome Study (ATOS), the largest and longest cohort study of people with heroin dependence in Australia, has followed 615 people since 2001. Participants have since been followed up at six time points, most recently 18–20 years after recruitment.4
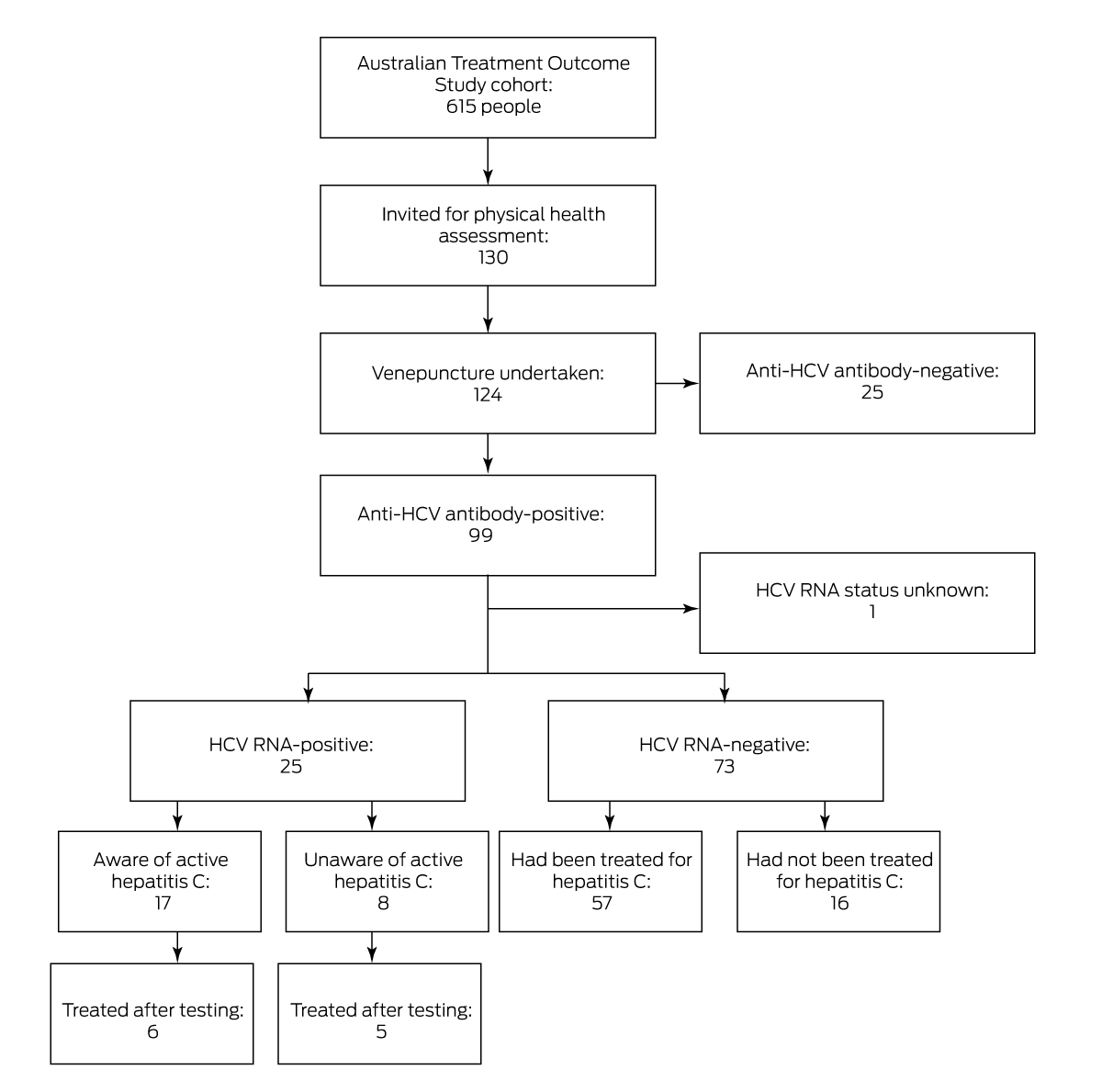
At the most recent follow‐up, we invited a convenience sample of 130 participants to attend Royal Prince Alfred Hospital (RPAH) in Sydney during 1 September 2019 – 30 April 2021 for physical health assessments, including taking of a medical history, physical examination, and blood tests. Prior to being tested for HCV infection, participants were asked about their hepatitis C status, their most recent visit to a primary health care provider, whether they had a regular primary health care provider, and whether their regular provider was their opioid treatment program prescriber. Serum samples were tested for anti‐HCV antibodies; if positive, polymerase chain reaction testing for HCV RNA was undertaken. Hepatitis C status was defined as active (HCV RNA‐positive) or past infection (HCV RNA‐negative, anti‐HCV antibody‐positive), not exposed (anti‐HCV antibody‐negative), or unknown. All participants also underwent liver elastography, and liver stiffness was graded according to Australian guidelines.5
Participants were informed of their positive hepatitis C status either directly (by telephone) or via letters to their primary health care providers, and encouraged to seek treatment. Two years later, we looked for evidence in their electronic health records of anti‐viral prescribing or follow‐up serological testing for HCV. Statistical analyses were undertaken in SPSS 27 (IBM). The Sydney Local Health District Human Research Ethics Committee (RPAH zone) approved the 18–20‐year follow‐up study (X18‐0512; HREC/18/RPAH/733).
Of the 130 invited ATOS participants who consented to HCV testing (95%), blood was successfully collected from 124. A total of 99 people had been exposed to HCV (80%): 25 had active hepatitis (none of whom had sought treatment), 73 had infections that had since resolved, and the HCV RNA status of one participant could not be determined. As 57 of participants with past infections had been treated (78%), 57 of the 99 people exposed to HCV had been treated (58%); past infections in sixteen people had apparently resolved without treatment (Box 1, Box 2).
Seventeen of 25 people with active HCV infections (68%) were aware of their status prior to testing, including eight in opioid treatment programs (32%), which require at least quarterly reviews by a prescribing clinician.6 Liver elastography indicated cirrhosis in two people with active infections, and fibrosis in four others.
Twenty‐three of 25 people with active HCV infections (92%) or (in eighteen cases) their general practitioners could be contacted to inform them about their status. Fourteen of 25 people (56%) did not commence treatment during the two years following testing, including eleven of the seventeen aware of their infection prior to testing. HCV infection had cleared in four of the eleven people who did commence treatment, which was prescribed by their opioid treatment program prescriber (four participants), general practitioner (five), or study doctor (one); one participant was not contacted about their status, but had commenced treatment in prison. Five of the eight people who had been unaware of their HCV infections commenced antiviral therapy within two years, including all three who did not believe they were infected.
At the time of HCV testing, 100 of 130 participants had seen their primary health care practitioner during the preceding six months (77%). The median time from medical review was similar for people with (30 days; interquartile range [IQR], 18–277 days) or without active hepatitis C infections (31 days; IQR, 12–151 days). The likelihood of active hepatitis C was higher for participants who did not have regular general practitioners or whose primary physician was their opioid treatment program prescriber than for those with regular general practitioners who were not their opioid treatment program prescribers (15 of 51 [29%] v 10 of 79 [13%]; OR, 2.9; 95% CI, 1.2–7.0).
The hepatitis C treatment rate (59%) we report exceeds the estimated overall rate among people in Australia with HCV infections (40–45%).7 All but two participants with active hepatitis C infections, or their general practitioners, had been informed about their status, but treatment may not have been initiated because of general practitioner uncertainty about treatment, or because the participant did not see their primary health care practitioner after testing. However, the fact that fourteen of 25 people with active infections did not commence treatment within two years, including eleven who had been aware of their status prior to testing, suggests that treatment hesitancy may be a factor in reducing treatment rates.
In conclusion, testing people at risk of HCV infection should be better integrated into opioid treatment programs4 if eradication is to be achieved by 2030.
Box 1 – Assessment of hepatitis C status of participants in the Australian Treatment Outcome Study (ATOS) 18–20‐year follow‐up, 1 September 2019 – 30 April 2021
Box 2 – Demographic and clinical information for 130 participants in the Australian Treatment Outcome Study (ATOS) 18–20‐year follow‐up, 1 September 2019 – 30 April 2021, by hepatitis C status
|
Characteristic |
All participants |
Active hepatitis C |
Past hepatitis C |
Not exposed |
Unknown |
||||||||||
|
|
|||||||||||||||
|
Participants |
130 |
25 |
73 |
25 |
7 |
||||||||||
|
Sex (men) |
84 (65%) |
17 (68%) |
46 (63%) |
16 (64%) |
5 (71%) |
||||||||||
|
Prior to HCV test |
|
|
|
|
|
||||||||||
|
Treated for HCV infection |
62 (48%) |
1 (4%) |
57 (78%) |
0 |
3 (43%) |
||||||||||
|
HCV infection known |
19 (15%) |
17 (68%) |
1 (1%) |
0 |
1 (14%) |
||||||||||
|
Time since health care practitioner review (days), median (IQR) |
31 (14–162) |
30 (18–277) |
31 (12–151) |
61 (18–316) |
31 (14–61) |
||||||||||
|
Primary physician |
|
|
|
|
|
||||||||||
|
General practitioner |
79 (61%) |
10 (40%) |
47 (64%) |
18 (72%) |
4 (57%) |
||||||||||
|
Opioid treatment program |
27 (21%) |
8 (32%) |
14 (19%) |
3 (12%) |
2 (29%) |
||||||||||
|
None |
24 (18%) |
7 (28%) |
12 (16%) |
4 (16%) |
1 (14%) |
||||||||||
|
Sought treatment after HCV study test |
— |
11 (44%) |
— |
— |
— |
||||||||||
|
|
|||||||||||||||
|
HCV = hepatitis C virus; IQR = interquartile range. |
|||||||||||||||
Received 8 February 2023, accepted 5 May 2023
- 1. Burnet Institute and Kirby Institute. Australia's progress towards hepatitis C elimination: annual report 2021. Melbourne: Burnet Institute; 2021. https://www.burnet.edu.au/media/wild4veh/burnetkirby‐hepc‐2021‐report.pdf (viewed June 2023).
- 2. Scott N, Sacks‐Davis R, Wade AJ, et al. Australia needs to increase testing to achieve hepatitis C elimination. Med J Aust 2020; 212: 365‐370. https://www.mja.com.au/journal/2020/212/8/australia‐needs‐increase‐testing‐achieve‐hepatitis‐c‐elimination
- 3. Coupland H, Day C, Haber P, et al. Client resistance to hepatitis C treatment initiation in opioid agonist treatment clinics in Sydney, Australia: a qualitative study. Drug Alcohol Rev 2022; 41: 706‐714.
- 4. Marel C, Wilson J, Darke S, et al. Patterns and predictors of heroin use, remission, and psychiatric health among people with heroin dependence: key findings from the 18–20‐year follow‐up of the Australian Treatment Outcome Study (ATOS). Int J Ment Health Addict 2023; doi: https://doi.org/10.1007/s11469‐022‐01006‐6 [online ahead of print].
- 5. Gastroenterological Society of Australia. Australian recommendations for the management of hepatitis C virus infection: a consensus statement (2022). Melbourne: Gastroenterological Society of Australia, 2022. https://www.hepcguidelines.org.au/wp‐content/uploads/2023/02/hepatitis‐C‐virus‐infection‐a‐consensus‐statement‐2022‐100223.pdf (viewed Apr 2023).
- 6. NSW Ministry of Health. NSW clinical guidelines: treatment of opioid dependence: 2018. Sept 2018. https://www.health.nsw.gov.au/aod/Publications/nsw‐clinical‐guidelines‐opioid.pdf (viewed Jan 2023).
- 7. Burnet Institute; Kirby Institute. Australia's progress towards hepatitis C elimination: annual report 2020. Melbourne: Burnet Institute, 2020. https://kirby.unsw.edu.au/sites/default/files/kirby/report/Australias_progress_towards_hepatitis_C_elimination%E2%80%93Annual_Report_2020.pdf (viewed June 2023).






Correspondence: ctre9656@uni.sydney.edu.au
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
This investigation was supported by a National Health and Medical Research Council (NHMRC) project grant (APP1147212). Christina Marel, Maree Teesson, and Katherine Mills are supported by NHMRC fellowships. The project was also supported by the Matilda Centre for Research in Mental Health and Substance Use, University of Sydney. Paul Haber is supported by a Medical Research Future Fund fellowship.
We thank Susan Anderson (Sydney Local Health District Drug Health Services, Edith Collins Centre for Translational Research) for her assistance with data collection; Jack Wilson, Katherine Haasnoot, Rachel Visontay, and Madeleine Keaveny (Matilda Centre for Research in Mental Health and Substance Use, University of Sydney) for organising the interviews; and Shane Darke (National Drug and Alcohol Research Centre, University of New South Wales), Joanne Ross (NSW Ministry of Health; National Drug and Alcohol Research Centre, University of New South Wales), and Tim Slade (Matilda Centre for Research in Mental Health and Substance Use, University of Sydney) for their participation in establishing the ATOS study.
No relevant disclosures.