The known: Small cell lung cancer (SCLC) is an aggressive disease with poor survival outcomes.
The new: Active treatment was provided to 90% of Victorians with newly diagnosed SCLC, initiated within fourteen days of diagnosis for 72% of treated patients. The proportions of cases discussed at multidisciplinary meetings (55%) and of patients who underwent supportive care screening (37%) or were referred for palliative care (39%) were, however, suboptimal, as was documentation of performance status (66%).
The implications: Defining SCLC‐specific quality of care indicators for a national data registry could reduce unwarranted variations in care equity, access, management, and outcomes in a learning health system.
Small cell lung cancers (SCLC) comprise 10–14% of all lung cancers. They are characterised by a rapid doubling time, a high growth fraction, and the early development of widespread metastases.1,2 Most patients present with stage IV (extensive stage) disease, and median survival time without systemic therapy is 2–4 months.3,4 Standard staging evaluation includes computed tomography (CT) imaging of the thorax and abdomen, CT or magnetic resonance imaging (MRI) of the brain, and fluorodeoxyglucose–positron emission tomography (FDG‐PET). SCLC is amenable to chemotherapy and radiotherapy, but recurrence rates are high and SCLC is often resistant to subsequent lines of treatment, leading to a 5‐year survival rate of less than 10%.5
Chemotherapy has been the backbone of SCLC treatment for twenty years.6,7 The results of randomised controlled trials of radiotherapy dominated practice until the emergence of immunotherapy agents.8,9 The recent confirmation of the efficacy of immune checkpoint inhibitors such as atezolizumab and durvalumab has led to their adoption alongside chemotherapy for first line treatment of stage IV disease10,11,12 (listed in the Pharmaceutical Benefit Scheme, March 202013).
Guideline‐recommended treatment for limited stage (stages I–III) SCLC with good performance status is concurrent chemo‐radiotherapy and, for relatively rare stage I disease, surgical resection or stereotactic ablative radiotherapy with adjuvant chemotherapy. Additional adjuvant management includes prophylactic cranial irradiation or brain MRI surveillance. If performance status is poor (Eastern Co‐operative Oncology Group [ECOG] grades 2–4) because of SCLC, sequential chemotherapy with or without radiotherapy is recommended. For extensive stage (stage IV) disease with good performance status or poor performance status because of SCLC, treatment includes combination systemic therapy, including chemotherapy and immunotherapy, adjuvant thoracic radiotherapy, and palliative radiotherapy at symptomatic sites. When performance status is poor for reasons other than SCLC (disease of any stage), individualised and supportive care is recommended. Follow‐up, including survivorship care planning, imaging surveillance, and smoking cessation, is integral to holistic care.7
Only limited information on guideline adherence, patterns of care, and survival outcomes for people with SCLC are available.14 A recent systematic review found that treatment options were limited and overall survival poor, but data were fragmented and inconsistently reported.15 Unwarranted variation in care and important outcomes have been reported in Australia,16,17 particularly for people in lower socio‐economic status areas18 or rural areas,19 as well as for Aboriginal and Torres Strait Islander people.20
Evaluations of the quality of SCLC care should be based on disease‐specific outcomes related to safe, timely, evidence‐based and multidisciplinary, guideline‐concordant treatment, patient‐reported experience of care, and accessibility of care. Purpose‐designed clinical quality registries can report key performance process and outcome measures in a timely manner, providing evidence of clinical performance in a learning health system.21
Pattern of care studies are an important element of practice evaluation and health service research because they identify disparities in health care that affect outcomes,22 stimulating quality improvement and innovations that reduce disparities in health care. We therefore investigated stage‐specific patterns of treatment and the influence of management and treatment provision on survival rates for people newly diagnosed with SCLC in Victoria.
Methods
We analysed data provided by the Victorian Lung Cancer Registry (VLCR), a clinical quality registry that systematically collects process and outcomes data for all people with newly diagnosed lung cancer in Victoria.23 We included patient characteristics, diagnostic method, treatment type (chemotherapy, radiotherapy, surgery), and survival time data for all people diagnosed with SCLC at nineteen Victorian health services and fifty hospitals during 11 April 2011 – 18 December 2019, with follow‐up to 5 March 2020.
Definitions
For SCLC staging, we used the International Association for the Study of Lung Cancer (IASLC) system,24 respectively combining tumour node metastasis (TNM) stages I–III and IV with the older limited and extensive stage disease categories. Performance status was assessed with the ECOG scale for capacity for self‐care.25 Overall survival was defined as time from date of diagnosis to date of death.
Statistical analysis
We summarise categorical data as numbers and proportions, normally distributed continuous data as means with standard deviation (SDs), and non‐normally distributed continuous data as medians with interquartile ranges (IQRs). For categorical variables, the statistical significance of between‐group differences was assessed in Fisher exact tests (when the expected frequency was lower than five per cell) or χ2 tests. The statistical significance of between‐group differences for continuous variables was assessed in Student t tests (normally distributed data) or Mann–Whitney U tests (non‐normally distributed data). P < 0.05 was deemed statistically significant. Time from diagnosis to death was analysed in Kaplan–Meier survival analyses. Statistical analyses were performed in Stata 16.1.
Associations between treatments and demographic characteristics were assessed in logistic regression models and reported as odds ratios (ORs) with 95% confidence intervals (CIs). Associations between treatment and survival were assessed in Cox proportional hazards regression models and reported as hazard ratios (HRs) with 95% CIs.
Ethics approval
The Victorian Lung Cancer Registry is administered by the Monash University Department of Epidemiology and Preventative Medicine (National Mutual Acceptance ethics approval: HREC/16/Alfred/84) and managed in a governance structure based on Australian Committee on Safety and Quality in Healthcare principles.26 The study reported in this article was approved as Monash University project 26764 (to October 2025).
Results
During 11 April 2011 – 18 December 2019, 1006 people were diagnosed with SCLC (10.4% of 9630 lung cancer diagnoses in the VLCR). Their median age was 69 years (IQR, 62–77 years), 429 were women (43%); 452 were current smokers (45%) and 469 former smokers (47%). Clinical stage was defined for 896 people (89%): TNM stages I–III, 268 (30%); TNM stage IV, 628 (70%). A total of 481 people had no other recorded medical conditions (48%); ECOG performance status was grade 0 or 1 for 489 patients at diagnosis (47%), and histological or cytological confirmation was available for 938 diagnoses (93%). PET scans were recorded for 426 people (42%) (Box 1).
Cancer management
The cases of 552 patients had been discussed at multi‐disciplinary meetings (55%) (Box 2). Presentations were less likely if the person was initially referred from a private hospital (26 of 124, 21% v from public hospitals 526 of 882, 60%; OR, 0.18; 95% CI, 0.11–0.28). A total of 377 people (37%) had received supportive care screening; 40 people with stage I–III disease (15%) and 295 with stage IV disease had been referred for palliative care (47%) (Box 2).
Treatment
Eight hundred and ninety‐one people received active treatment (89%): 843 received chemotherapy (84%), 460 radiotherapy (46%), and 419 both chemotherapy and radiotherapy (42%); 23 had undergone surgery (including seven who did not receive other treatment) (2%) (Box 2). Treatment had commenced within fourteen days of diagnosis for 632 of 875 patients for whom timing information was available (72%) (Box 3).
The median time from diagnosis to chemotherapy initiation was eight days (IQR, 5–18 days); the interval was less than fourteen days for 590 of 842 people for whom initiation time was known (70%). Chemotherapy regimens included guideline‐concordant treatment with carboplatin/etoposide (697 people, 83%), cisplatin/etoposide (102, 12%), carboplatin/gemcitabine (eleven, 1%), or carboplatin/paclitaxel (ten, 1%). Chemotherapy was less likely for people aged 65 years or more (553 of 669 people [83%] v 290 of 312 people under 65 years of age [93%]; OR, 0.93; 95% CI, 0.91–0.95).
Of the 460 people who received radiotherapy, 419 also received chemotherapy (91%). Total prescribed dose was not consistently reported. Dose fractionation was daily for 270 patients (59%), single fraction for 46 (10%), twice daily for 36 (8%), stereotactic body radiotherapy for ten (2%), and unknown for 98 (21%). Radiotherapy was less likely for people aged 60 years or more (360 of 805 people [45%] v 100 of 176 people under 60 years of age [57%]; OR, 0.96; 95% CI, 0.95–0.97) and people diagnosed in private hospitals (45 of 120 people [38%] v 415 of 861 diagnosed in public hospitals [48%]; OR, 0.64; 95% CI 0.43–0.96); it was more likely for people diagnosed in regional hospitals (140 of 252 people [56%] v 320 of 729 diagnosed in metropolitan hospitals [44%]; OR, 1.60; 95% CI, 1.20–2.13). Chemotherapy and radiotherapy were less likely for people with poorer performance status (ECOG grade 2–4: 70 of 171 [41%] v ECOG grade < 2: 268 of 480 [56%]; OR, 1.89; 95% CI, 1.43–2.48) or aged 70 years or more (166 of 495 [34%] v 253 of 511 people under 70 years [50%]; OR, 0.51; 95% CI, 0.40–0.66).
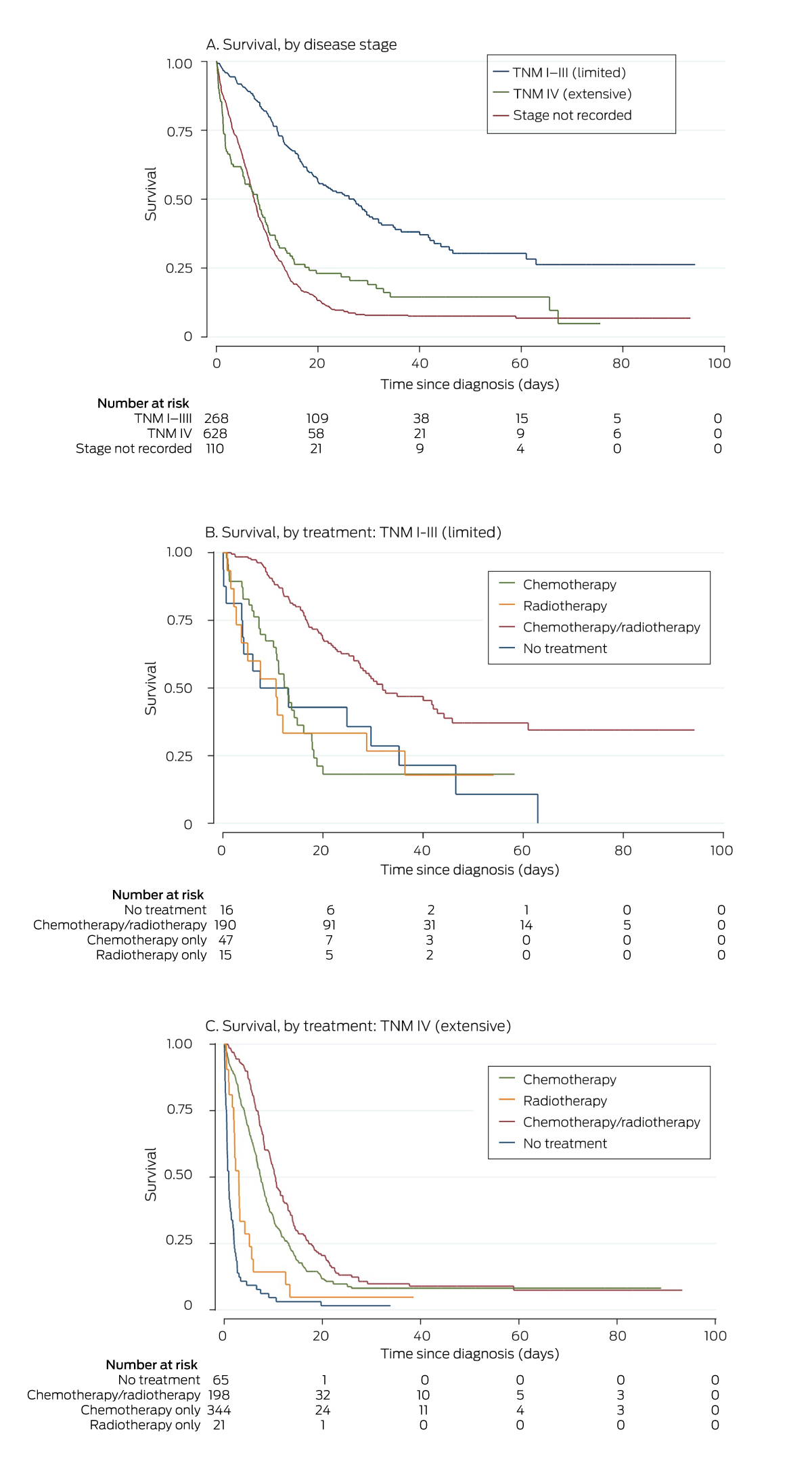
Of the 268 people with stage I–III SCLC, 239 received chemotherapy (89%) and 204 received radiotherapy (76%); 190 received combination chemo‐radiotherapy (71%), including 128 of 164 with ECOG grades 0 or 1 (78%) and 20 of 31 with ECOG grades 2–4 (65%). Surgical resection was undertaken with stage I–III SCLC (pneumonectomy, one; lobectomy, seven, segmentectomy, three; wedge resection, three); video‐assisted thoracic surgery was undertaken in twelve patients, and open surgery in two. Nine patients who had surgery had not received systemic anti‐cancer therapy.
Of the 628 patients with stage IV SCLC, 559 received chemotherapy (89%), including 273 of 288 with ECOG < 2 (95%) and 96 of 123 with ECOG 2–4 (78%); 220 received radiotherapy (35%) and 201 both chemotherapy and radiotherapy (32%).
Survival
A total of 766 people died during the registry follow‐up period (76%), including 90 within 30 days of diagnosis (8.9%) (Box 4). Overall median survival time from diagnosis was 8.9 months (IQR, 4.2–16 months); it was 16.3 (IQR, 9.3–30) months for people with stage I–III SCLC, and 7.2 (IQR, 3.3–12) months for those with stage IV disease. Overall survival declined with age (per year: HR, 1.03; 95% CI, 1.02–1.03), and was poorer for people with ECOG grades of 3 or 4 (v no recorded status: HR, 2.40; 95% CI, 1.80–3.20), and for those not receiving treatment (HR, 3.00; 95% CI, 2.44–3.67). Overall survival was higher for people whose cases were presented to a multidisciplinary meeting (HR, 0.66; 95% CI, 0.58–0.77), those who received multimodality treatment (HR, 0.42; 95% CI, 0.36–0.49), and for people who received chemotherapy within fourteen days of diagnosis (HR, 0.68; 95% CI, 0.48–0.94) (Box 5). For patients who received chemotherapy, the median survival time (9.6 [IQR, 5.7–17] months) was longer than for those who did not (2.0 [IQR 0.6–9.8] months). Median survival for 559 people with stage IV cancer treated with chemotherapy (eight [IQR, 4.9–13] months) was longer than for the 69 who were not (1.0 [IQR, 0.5–3.0] months).
Discussion
We report information about patterns of care for a large group of people with SCLC. Of 1006 cases diagnosed in Victoria over eight and a half years, 628 people presented with stage IV disease, or 70% of those for whom staging information was available, similar to the 71% reported by an American study.27 The overall chemotherapy treatment rate was high (84%; 89% for patients with staged disease), 71% of people with TNM I–III disease had received guideline‐concordant chemo‐radiotherapy, and treatment had been initiated within thirty days of diagnosis for 92% of patients. Performance status was not documented at diagnosis for 34% of people, and TNM staging for 11%; only 55% of cases were discussed at multidisciplinary meetings, and only 39% of patients were referred for palliative care.
Survival was significantly higher for people who commenced chemotherapy within fourteen days of diagnosis (overall: 32%), but chemotherapy was initiated within this timeframe for only 59% of patients. The reported impact of timeliness of care on outcomes for people with lung cancer is inconsistent,28 and information regarding its importance for the treatment of people with SCLC is limited.
Emotional distress has been described as the sixth vital sign in oncology,29 and overseas guidelines recommend longitudinal screening for distress during cancer treatment.2 However, only 37% of patients in our study underwent supportive care screening. The availability of lung cancer nurse specialists is very limited in Australia; the Australian Lung Foundation recently reported that twelve full‐time equivalent nurse specialists are available30 to manage the 13 810 people who receive new lung cancer diagnoses each year.31 Lung cancer nurse specialists could make major contributions to care by responding to patient distress, enhancing patient characterisation, improving communication with medical specialists and care coordination, and helping people navigate medical and social care pathways.
PET is the recommended SCLC staging modality for all people undergoing treatment with curative intent, but PET scans were recorded for only 42% of patients in our study. A 2012 systematic review found that PET‐based staging can change management for 28% of patients with SCLC, increase the provision of life‐prolonging radiotherapy, and avert unwarranted radiotherapy, thereby reducing unnecessary physical toxicity and expense.32 PET scanning is not subsidised by Medicare for people with SCLC, and this is a major obstacle to best practice staging and reducing treatment costs for patients.
Optimal provision of appropriate active cancer treatment is crucial to maximising survival. Only 71% of people with limited stage (TNM I–III) SCLC received concurrent chemo‐radiotherapy, despite its being the mainstay of treatment for patients with limited stage disease. Immune checkpoint inhibitors became available during the period covered by our study, and more detailed collection of oncologic treatment details (systemic anti‐cancer therapy, radiotherapy, clinical trial participation) is needed.
Reporting of patterns of care for people with SCLC would be substantially enhanced by a purpose‐built clinical dataset effectively linked with the Victorian Cancer Registry and administrative datasets. Such data linkage would support assessment of equity of outcomes, identify research and clinical performance deficits, and drive innovation in practice.33 As the Australian SCLC clinical practice guidelines34 are somewhat fragmentary, we propose an updated panel of quality indicators for confirmation in a national Delphi process (Box 6).
Limitations
Our study included the largest reported Australian cohort of consecutive patients with SCLC, and we analysed prospectively collected data linked with complete survival data. However, registry studies are subject to unmeasured confounding related to inconsistent data collection for variables such as biomarkers, patient frailty, treatment tolerance, and patient preferences; comprehensive treatment details are not recorded, nor assessments of patients’ experience of treatment and satisfaction. As the VLCR collects data on about 85% of all new Victorian lung cancer cases, selection bias is possible, as is information bias due to loss to follow‐up caused by patient transfer between non‐participating institutions; more comprehensive data linkage is needed. Further, the VLCR captures data on first‐line treatment, but information on disease‐free survival, disease recurrence, and subsequent lines of treatment is less complete. As there is no consensus about second‐line treatment of people with SCLC, this limitation is probably of minor consequence.
Conclusions
Our patterns of care study provides evidence of deficits in the best practice management in the treatment of people with SCLC in Victoria. We identified opportunities for improving the level of nursing care, clinical characterisation of patents, supportive care screening, multidisciplinary meeting evaluation, and palliative care referral. A national registry of SCLC‐specific management and outcomes data could improve the quality and safety of care in a learning health system.
Box 1 – Demographic and clinical characteristics of 1006 people diagnosed with small cell lung cancers in Victoria, 11 April 2011 – 18 December 2019
|
Characteristic |
Value |
||||||||||||||
|
|
|||||||||||||||
|
Total number of people |
1006 |
||||||||||||||
|
Age at diagnosis (years), median (IQR) |
69 (62–77) |
||||||||||||||
|
Sex (women) |
429 (43%) |
||||||||||||||
|
Smoking history |
|
||||||||||||||
|
Current smoker |
452 (45%) |
||||||||||||||
|
Ex‐smoker |
469 (47%) |
||||||||||||||
|
Non‐smoker |
19 (2%) |
||||||||||||||
|
Not recorded |
66 (7%) |
||||||||||||||
|
Aboriginal or Torres Strait Islander people |
13 (1%) |
||||||||||||||
|
Other medical conditions |
|
||||||||||||||
|
Diabetes |
212 (21%) |
||||||||||||||
|
Renal insufficiency |
25 (2%) |
||||||||||||||
|
Myocardial infarction |
167 (17%) |
||||||||||||||
|
Respiratory condition |
161 (16%) |
||||||||||||||
|
Neoplastic condition |
152 (15%) |
||||||||||||||
|
None |
481 (48%) |
||||||||||||||
|
Weight loss at time of diagnosis |
|
||||||||||||||
|
Yes |
449 (45%) |
||||||||||||||
|
No |
315 (31%) |
||||||||||||||
|
Not recorded |
242 (24%) |
||||||||||||||
|
ECOG performance status at diagnosis |
|
||||||||||||||
|
0 or 1 |
489 (49%) |
||||||||||||||
|
2–4 |
174 (17%) |
||||||||||||||
|
Not recorded |
343 (34%) |
||||||||||||||
|
Disease stage |
|
||||||||||||||
|
TNM I–III (limited stage) |
268 (27%) |
||||||||||||||
|
TNM IV (extensive stage) |
628 (62%) |
||||||||||||||
|
Not staged |
110 (11%) |
||||||||||||||
|
Most secure basis for diagnosis |
|
||||||||||||||
|
Clinical |
43 (4.3%) |
||||||||||||||
|
Histology or cytology |
938 (93%) |
||||||||||||||
|
Death certificate |
2 (0.2%) |
||||||||||||||
|
Unknown |
23 (2%) |
||||||||||||||
|
PET scan undertaken |
426 (42%) |
||||||||||||||
|
Diagnosed within 28 days of referral* |
735/896 (83%) |
||||||||||||||
|
Commenced treatment within 14 days of diagnosis* |
632/875 (72%) |
||||||||||||||
|
Commenced treatment within 42 days of referral* |
509/781 (65%) |
||||||||||||||
|
Chemotherapy: location of hospital |
|
||||||||||||||
|
Metropolitan Melbourne |
591 (71%) |
||||||||||||||
|
Regional Victoria |
242 (29%) |
||||||||||||||
|
Not recorded |
173 |
||||||||||||||
|
Chemotherapy: type of hospital |
|
||||||||||||||
|
Public hospital |
719 (86%) |
||||||||||||||
|
Private hospital |
114 (14%) |
||||||||||||||
|
Not recorded |
173 |
||||||||||||||
|
|
|||||||||||||||
|
ECOG = Eastern Cooperative Oncology Group Performance Status; IQR = interquartile range; PET = positron emission tomography; TNM = tumour node metastasis. * Based on cases for which timeliness data were available. |
|||||||||||||||
Box 2 – Management and treatment of small cell lung cancer for 1006 people diagnosed with small cell lung cancers in Victoria, 11 April 2011 – 18 December 2019
|
|
|
Age (years), median (IQR) |
Stage |
|
|||||||||||
|
Management/treatment |
Number |
Received treatment |
Did not receive treatment |
TNM I–III |
TNM IV |
Not recorded |
P* |
||||||||
|
|
|||||||||||||||
|
Total number of people |
1006 |
|
|
268 |
628 |
110 |
|
||||||||
|
Chemotherapy |
843 (84%) |
68 (61–75) |
76 (68–80) |
239 (89%) |
559 (89%) |
68 (62%) |
0.87 |
||||||||
|
Radiotherapy |
460 (46%) |
67 (60–74) |
71 (64–78) |
204 (76%) |
220 (35%) |
38 (35%) |
< 0.001 |
||||||||
|
Chemotherapy and radiotherapy |
419 (42%) |
67 (60–73) |
71 (64–78) |
190 (71%) |
201 (32%) |
31 (28%) |
< 0.001 |
||||||||
|
Surgery |
23 (2%) |
71 (65–78) |
69 (62–77) |
14 (5%) |
6 (1%) |
4 (4%) |
< 0.001 |
||||||||
|
No active treatment |
115 (11%) |
69 (62–76) |
76 (68–82) |
11 (4%) |
63 (10%) |
38 (35%) |
< 0.001 |
||||||||
|
Multidisciplinary meeting discussion |
552 (55%) |
69 (62–76) |
70 (62–78) |
204 (76%) |
308 (49%) |
41 (37%) |
< 0.001 |
||||||||
|
Supportive care screening |
377 (37%) |
68 (61–75) |
70 (63–78) |
131 (49%) |
220 (35%) |
27 (25%) |
< 0.001 |
||||||||
|
Palliative care referral |
388 (39%) |
71 (64–78) |
68 (61–75) |
40 (15%) |
295 (47%) |
65 (59%) |
< 0.001 |
||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; TNM = tumour node metastasis. * Proportions of people with TNM I–III v TNM IV stage disease. |
|||||||||||||||
Box 3 – Time from diagnosis to initiation of active treatment for 875 people diagnosed with small cell lung cancers in Victoria, 11 April 2011 – 18 December 2019, for whom treatment initiation dates were available
|
Treatment |
People |
Median time in days (IQR) |
< 14 days |
< 30 days |
30 days or more |
||||||||||
|
|
|||||||||||||||
|
Any active treatment |
875 |
8 (4–16) |
632 (72%) |
801 (92%) |
74 (8%) |
||||||||||
|
Chemotherapy |
842 |
8 (5–18) |
609 (72%) |
753 (89%) |
89 (11%) |
||||||||||
|
Radiotherapy |
119 |
40 (29–53) |
6 (5%) |
38 (32%) |
81 (68%) |
||||||||||
|
Surgery |
20 |
0 (0–5) |
17 (85%) |
19 (95%) |
1 (5%) |
||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
Box 4 – Kaplan–Meier analysis of survival for 1006 people diagnosed with small cell lung cancers in Victoria, 11 April 2011 – 18 December 2019, by TNM disease stage (A) and by treatment type (B, C)
Box 5 – Survival analysis for 1006 people diagnosed with small cell lung cancers in Victoria, 11 April 2011 – 18 December 2019, by stage: univariate analyses
|
|
Disease stage: hazard ratio (95% confidence interval) |
||||||||||||||
|
Characteristic |
All stages |
TNM I–III |
TNM IV |
Not staged |
|||||||||||
|
|
|||||||||||||||
|
Age (per year) |
1.03 (1.02–1.03) |
1.03 (1.01–1.05) |
1.03 (1.02–1.04) |
1.02 (1.04–1.05) |
|||||||||||
|
Sex (men) |
1.15 (0.99–1.33) |
1.07 (0.76–1.49) |
1.09 (0.92–1.30) |
1.09 (0.72–1.64) |
|||||||||||
|
ECOG performance status grade (v no recorded status) |
|
|
|
|
|||||||||||
|
0 or 1 |
0.68 (0.58–0.79) |
1.03 (0.70–1.51) |
0.61 (0.50–0.74) |
0.77 (0.46–1.26) |
|||||||||||
|
2 |
1.16 (0.92–1.47) |
1.14 (0.58–2.23) |
0.99 (0.76–1.30) |
1.75 (0.87–3.52) |
|||||||||||
|
3 or 4 |
2.40 (1.80–3.20) |
2.29 (1.02–5.15) |
2.72 (1.95–3.81) |
1.87 (0.84–4.16) |
|||||||||||
|
Regional hospital |
1.04 (0.89–1.23) |
0.85 (0.56–1.29) |
0.99 (0.82–1.21) |
1.18 (0.77–1.80) |
|||||||||||
|
Private hospital |
1.17 (0.95–1.44) |
1.09 (0.66–1.79) |
1.23 (0.96–1.57) |
1.00 (0.58–1.70) |
|||||||||||
|
Treatment |
|
|
|
|
|||||||||||
|
Multimodality treatment |
0.42 (0.36–0.49) |
0.36 (0.25–0.50) |
0.60 (0.50–0.72) |
0.67 (0.42–1.07) |
|||||||||||
|
Multidisciplinary meeting |
0.66 (0.58–0.77) |
1.23 (0.83–1.81) |
0.68 (0.58–0.81) |
1.02 (0.66–1.58) |
|||||||||||
|
No treatment |
3.00 (2.44–3.67) |
2.96 (1.55–5.63) |
5.42 (4.15–7.11) |
1.52 (0.99–2.36) |
|||||||||||
|
Chemotherapy within 14 days of diagnosis |
0.68 (0.48–0.94) |
0.68 (0.49–0.95) |
0.78 (0.66– 0.93) |
1.00 (0.64–1.55) |
|||||||||||
|
|
|||||||||||||||
|
ECOG = Eastern Cooperative Oncology Group; TNM = Tumour Node Metastasis. |
|||||||||||||||
Box 6 – Proposed small cell lung cancer quality indicators
|
Patient group/quality indicator |
Evidence/ recommendation grades* |
||||||||||||||
|
|
|||||||||||||||
|
All disease stages |
|
||||||||||||||
|
Referral to lung cancer nurse specialist |
III B |
||||||||||||||
|
Supportive care screening |
III B |
||||||||||||||
|
Presentation of case to multidisciplinary meeting |
I A |
||||||||||||||
|
FDG‐PET scan to confirm stage |
II A |
||||||||||||||
|
Staging according to TNM 8th edition35 |
IV A |
||||||||||||||
|
Commence chemotherapy within two weeks of diagnosis |
— |
||||||||||||||
|
Limited (stages I–III) |
|
||||||||||||||
|
Brain imaging (preferably MRI) |
III A |
||||||||||||||
|
Combination platinum etoposide chemotherapy regimen (ECOG performance grades 0 or 1) |
I A |
||||||||||||||
|
Commence chemotherapy within two weeks of diagnosis |
— |
||||||||||||||
|
Concurrent chemo‐radiotherapy (ECOG performance grades 0 or 1) |
I A |
||||||||||||||
|
Commence radiotherapy with start of chemotherapy cycle 1 or 2 |
II A |
||||||||||||||
|
Radiotherapy dose fractionation: 45 Gray twice a day, 30 fractions |
I A |
||||||||||||||
|
People with stage III disease, ECOG performance grades 0 or 1, under 70 years of age, no disease progression after chemo‐radiotherapy: prophylactic cranial irradiation |
III B |
||||||||||||||
|
Extensive (stage IV) |
|
||||||||||||||
|
Atezolizumab or durvalumab together with platinum etoposide chemotherapy (ECOG performance grades 0 or 1) |
I A |
||||||||||||||
|
Combination platinum etoposide chemotherapy if immunotherapy contraindicated (ECOG performance grades 0 or 1) |
I A |
||||||||||||||
|
People with ECOG performance grades 0 or 1, under 75 years of age, no disease progression after chemotherapy or MRI surveillance: prophylactic cranial irradiation |
II B |
||||||||||||||
|
Palliative care referral within eight weeks of diagnosis |
I B |
||||||||||||||
|
|
|||||||||||||||
|
ECOG = Eastern Cooperative Oncology Group; FDG‐PET = fluorodeoxyglucose–positron emission tomography; MRI = magnetic resonance imaging; TNM = tumour node metastasis. * Levels of evidence (Roman numerals) and grades of recommendation (letters), based on the Infectious Diseases Society of America–United States Public Health Service grading system (Supporting Information, table). |
|||||||||||||||
Received 11 June 2022, accepted 2 May 2023
- Joanna Huang1
- Wasek Faisal2
- Margaret Brand3
- Shantelle Smith3
- Marliese Alexander4,5
- Lisa Briggs3
- Matthew Conron5,6
- Mary Duffy4
- Thomas John4
- David Langton3,7
- Jacqueline Lesage3
- Michael MacManus4
- Paul Mitchell8
- Inger Olesen9
- Phillip Parente10,11
- Jennifer Philip5
- Evangeline Samuel1,12
- Javier Torres13
- Craig R Underhill14,15
- John R Zalcberg1,3
- Susan Harden3,4
- Rob Stirling1,3,16
- 1 Alfred Health, Melbourne, VIC
- 2 Grampians Health, Ballarat, VIC
- 3 Monash University, Melbourne, VIC
- 4 Peter MacCallum Cancer Institute, Melbourne, VIC
- 5 The University of Melbourne, Melbourne, VIC
- 6 St Vincent's Hospital Melbourne, Melbourne, VIC
- 7 Peninsula Health, Melbourne, VIC
- 8 Olivia Newton‐John Cancer Centre at Austin Health, Melbourne, VIC
- 9 Andrew Love Cancer Centre, Barwon Health, Geelong, VIC
- 10 Eastern Health Clinical School, Monash University, Melbourne, VIC
- 11 Eastern Health, Melbourne, VIC
- 12 Latrobe Regional Hospital, Traralgon, VIC
- 13 Goulburn Valley Health, Shepparton, VIC
- 14 Albury Wodonga Health, Wodonga, NSW
- 15 The University of New South Wales, Sydney, NSW
- 16 Monash University Central Clinical School, Melbourne, VIC
Correspondence: robert.stirling@monash.edu
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
We thank the steering committee, staff, and investigators of the Victorian Lung Cancer Registry for supporting this investigation.
No relevant disclosures.
- 1. Simone CB, Bogart JA, Cabrera AR, et al. Radiation therapy for small cell lung cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol 2020; 10: 158‐173.
- 2. National Comprehensive Cancer Network. Small cell lung cancer (Clinical practice guidelines in oncology). Version 3.2023. 21 Dec 2022. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf (viewed Mar 2023).
- 3. Slotman B, Faivre‐Finn C, Kramer G, et al; EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small‐cell lung cancer. N Engl J Med 2007; 357: 664‐672.
- 4. van Meerbeeck JP, Fennell DA, De Ruysscher DKM. Small‐cell lung cancer. Lancet 2011; 378: 1741‐1755.
- 5. Dómine M, Moran T, Isla D, et al. SEOM clinical guidelines for the treatment of small‐cell lung cancer (SCLC) (2019). Clin Translat Oncol 2020; 22: 245‐255.
- 6. Früh M, De Ruysscher D, Popat S, et al; ESMO Guidelines Working Group. Small‐cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013; 24 (Suppl 6): vi99‐vi105.
- 7. Dingemans AC, Früh M, Ardizzoni A, et al; ESMO Guidelines Committee. Small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2021; 32: 839‐853.
- 8. Hiddinga BI, Raskin J, Janssens A, et al. Recent developments in the treatment of small cell lung cancer. Eur Respir Rev 2021; 30: 210079.
- 9. Grønberg BH, Killingberg KT, Fløtten Ø, et al. High‐dose versus standard‐dose twice‐daily thoracic radiotherapy for patients with limited stage small‐cell lung cancer: an open‐label, randomised, phase 2 trial. Lancet Oncol 2021; 22: 321‐331.
- 10. Horn L, Mansfield AS, Szczęsna A, et al; IMpower133 Study Group. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med 2018; 379: 2220‐2229.
- 11. Goldman JW, Dvorkin M, Chen Y, et al; CASPIAN investigators. Durvalumab, with or without tremelimumab, plus platinum‐etoposide versus platinum‐etoposide alone in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): updated results from a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2021; 22: 51‐65.
- 12. Landre T, Chouahnia K, Des Guetz G, et al. First‐line immune‐checkpoint inhibitor plus chemotherapy versus chemotherapy alone for extensive‐stage small‐cell lung cancer: a meta‐analysis. Ther Adv Med Oncol 2020; 12: 1758835920977137.
- 13. Pharmaceutical Benefits Scheme. Atezolizumab for extensive‐stage small cell lung cancer: analysis of predicted versus actual utilisation, September 2022. Updated 31 Mar 2023. https://www.pbs.gov.au/info/industry/listing/participants/public‐release‐docs/2022‐09/atezolizumab‐24‐month‐review‐DUSC‐PRD‐2022‐09 (viewed June 2023).
- 14. Cramer‐van der Welle CM, Schramel F, van Leeuwen AS, et al; Santeon SCLC Study Group. Real‐world treatment patterns and outcomes of patients with extensive disease small cell lung cancer. Euro J Cancer Care (Engl) 2020; 29: e13250.
- 15. Povsic M, Enstone A, Wyn R, et al. Real‐world effectiveness and tolerability of small‐cell lung cancer (SCLC) treatments: a systematic literature review (SLR). PLoS One 2019; 14: e0219622.
- 16. Yu XQ, O'Connell DL, Gibberd RW, Armstrong BK. Assessing the impact of socio‐economic status on cancer survival in New South Wales, Australia 1996–2001. Cancer Causes Control 2008; 19: 1383‐1390.
- 17. Moore SP, Green AC, Bray F, et al. Survival disparities in Australia: an analysis of patterns of care and comorbidities among Indigenous and non‐Indigenous cancer patients. BMC Cancer 2014; 14: 517.
- 18. Banham D, Roder D, Eckert M, et al; CanDAD Aboriginal Community Reference Group and other CanDAD Investigators. Cancer treatment and the risk of cancer death among Aboriginal and non‐Aboriginal South Australians: analysis of a matched cohort study. BMC Health Serv Res 2019; 19: 771.
- 19. Hall SE, Holman CD, Sheiner H. The influence of socio‐economic and locational disadvantage on patterns of surgical care for lung cancer in Western Australia 1982–2001. Aust Health Rev 2004; 27: 68‐79.
- 20. Cunningham J, Rumbold AR, Zhang X, Condon JR. Incidence, aetiology, and outcomes of cancer in Indigenous peoples in Australia. Lancet Oncol 2008; 9: 585‐595.
- 21. McNeil JJ, Evans SM, Johnson NP, Cameron PA. Clinical‐quality registries: their role in quality improvement. Med J Aust 2010; 192: 244‐245. https://www.mja.com.au/journal/2010/192/5/clinical‐quality‐registries‐their‐role‐quality‐improvement
- 22. Albain KS, de la Garza Salazar J, Pienkowski T, et al. Reducing the global breast cancer burden: the importance of patterns of care research. Clin Breast Cancer 2005; 6: 412‐420.
- 23. Stirling RG, Evans SM, McLaughlin P, et al. The Victorian Lung Cancer Registry pilot: improving the quality of lung cancer care through the use of a disease quality registry. Lung 2014; 192: 749‐758.
- 24. Nicholson AG, Chansky K, Crowley J, et al; Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of Small Cell Lung Cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 300‐311.
- 25. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649‐655.
- 26. Australian Commission on Safety and Quality in Health Care. Framework for Australian clinical quality registries. Mar 2014. https://www.safetyandquality.gov.au/sites/default/files/migrated/Framework‐for‐Australian‐Clinical‐Quality‐Registries.pdf (viewed June 2023).
- 27. Parsons HM, Harlan LC, Stevens JL, Ullmann CD. Treatment of small cell lung cancer in academic and community settings: factors associated with receiving standard therapy and survival. Cancer J 2014; 20: 97‐104.
- 28. Evans SM, Earnest A, Bower W, et al. Timeliness of lung cancer care in Victoria: a retrospective cohort study. Med J Aust 2016; 204: 75. https://www.mja.com.au/journal/2016/204/2/timeliness‐lung‐cancer‐care‐victoria‐retrospective‐cohort‐study
- 29. Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care [letter]. J Clin Oncol 2005; 23: 6440‐6441.
- 30. Australia Lung Foundation. Lung cancer scorecard 2018–2021: information paper. Oct 2021. https://lungfoundation.com.au/resources/lung‐cancer‐scorecard‐2020 (viewed Mar 2023).
- 31. Australian Institute of Health and Welfare. Cancer in Australia 2021 (Cat. no. CAN 144). 1 Dec 2021. https://www.aihw.gov.au/reports/cancer/cancer‐in‐australia‐2021/summary (viewed June 2023).
- 32. Ruben JD, Ball DL. The efficacy of PET staging for small‐cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol 2012; 7 1015‐1020.
- 33. Bergin RJ, Thomas RJ, Whitfield K, White V. Concordance between optimal care pathways and colorectal cancer care: identifying opportunities to improve quality and reduce disparities. J Eval Clin Pract 2020; 26: 918‐926.
- 34. Cancer Council Australia Lung Cancer Guidelines Working Party. Clinical practice guidelines for the treatment of lung cancer. Updated 18 Aug 2017. https://wiki.cancer.org.au/australia/Guidelines:Lung_cancer (viewed Mar 2023).
- 35. Detterbeck FC, Chansky K, Groome P, et al; IASLC Staging and Prognostic Factors Committee, advisory boards, and participating institutions. The IASLC Lung Cancer Staging Project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol 2016; 11:1433‐1446.






Abstract
Objectives: To report stage‐specific patterns of treatment and the influence of management and treatment type on survival rates for people newly diagnosed with small cell lung cancer (SCLC).
Design: Cross‐sectional patterns of care study; analysis of data prospectively collected for the Victorian Lung Cancer Registry (VLCR).
Setting, participants: All people diagnosed with SCLC in Victoria during 1 April 2011 – 18 December 2019.
Main outcome measures: Stage‐specific management and treatment of people with SCLC; median survival time.
Results: During 2011–19, 1006 people were diagnosed with SCLC (10.5% of all lung cancer diagnoses in Victoria); their median age was 69 years (interquartile range [IQR], 62–77 years), 429 were women (43%), and 921 were current or former smokers (92%). Clinical stage was defined for 896 people (89%; TNM stages I–III, 268 [30%]; TNM stage IV, 628 [70%]) and ECOG performance status at diagnosis for 663 (66%; 0 or 1, 489 [49%]; 2–4, 174 [17%]). The cases of 552 patients had been discussed at multidisciplinary meetings (55%), 377 people had received supportive care screening (37%), and 388 had been referred for palliative care (39%). Active treatment was received by 891 people (89%): chemotherapy, 843 (84%); radiotherapy, 460 (46%); chemotherapy and radiotherapy, 419 (42%); surgery, 23 (2%). Treatment had commenced within fourteen days of diagnosis for 632 of 875 patients (72%). Overall median survival time from diagnosis was 8.9 months (IQR, 4.2–16 months; stage I–III: 16.3 [IQR, 9.3–30] months; stage IV: 7.2 [IQR, 3.3–12] months). Multidisciplinary meeting presentation (hazard ratio [HR], 0.66; 95% CI, 0.58–0.77), multimodality treatment (HR, 0.42; 95% CI, 0.36–0.49), and chemotherapy within fourteen days of diagnosis (HR, 0.68; 95% CI, 0.48–0.94) were each associated with lower mortality during follow‐up.
Conclusion: Rates of supportive care screening, multidisciplinary meeting evaluation, and palliative care referral for people with SCLC could be improved. A national registry of SCLC‐specific management and outcomes data could improve the quality and safety of care.